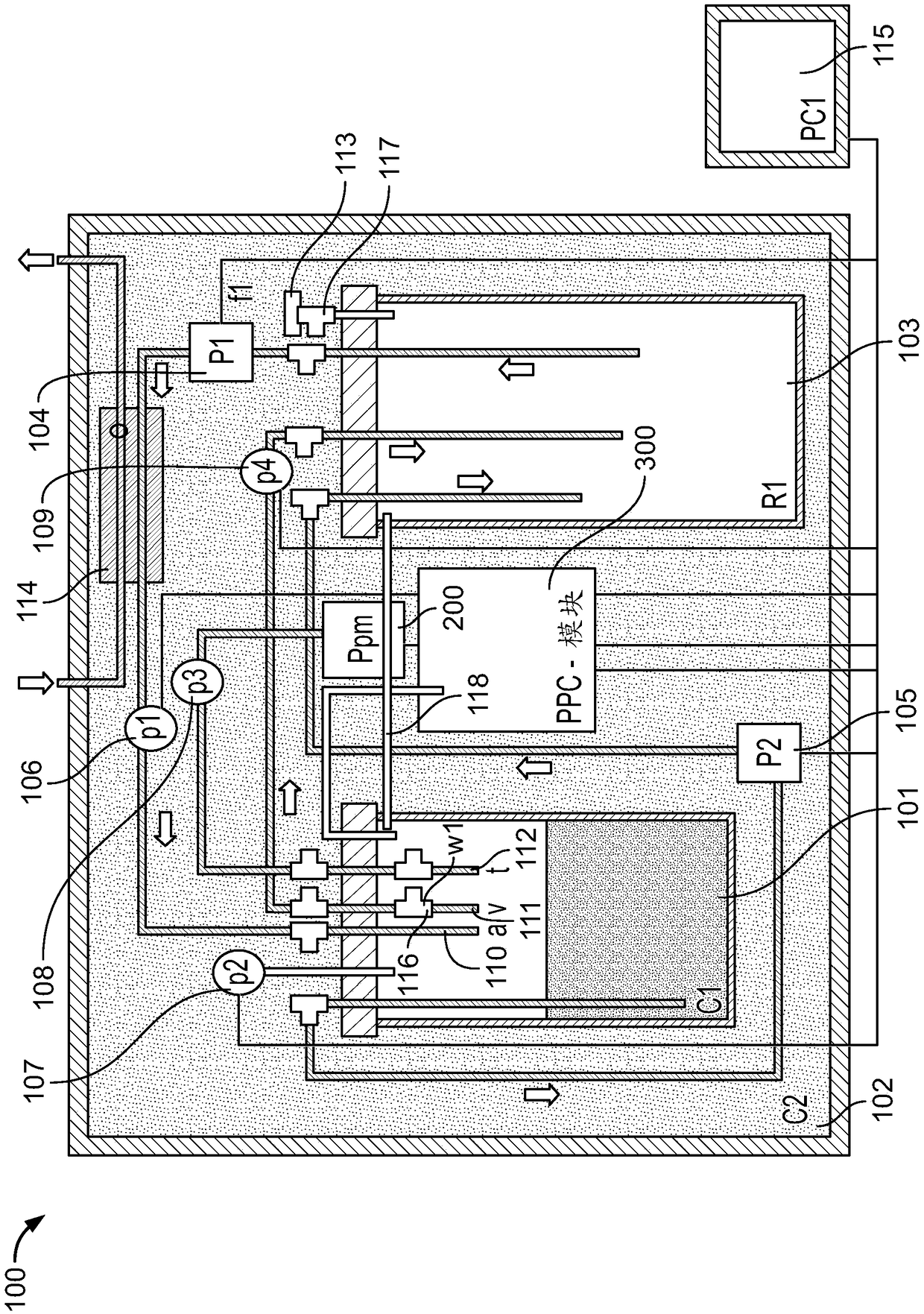
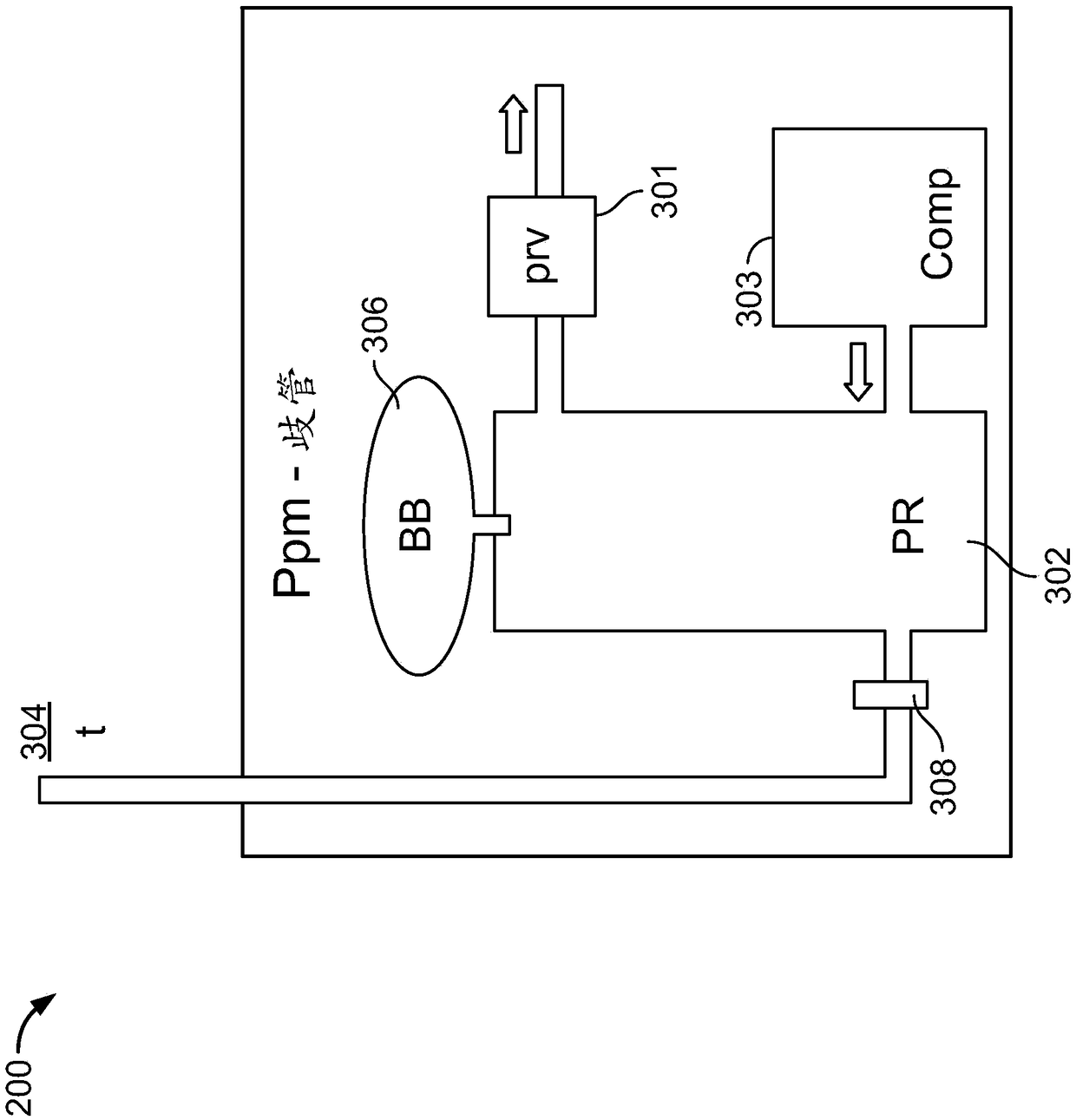
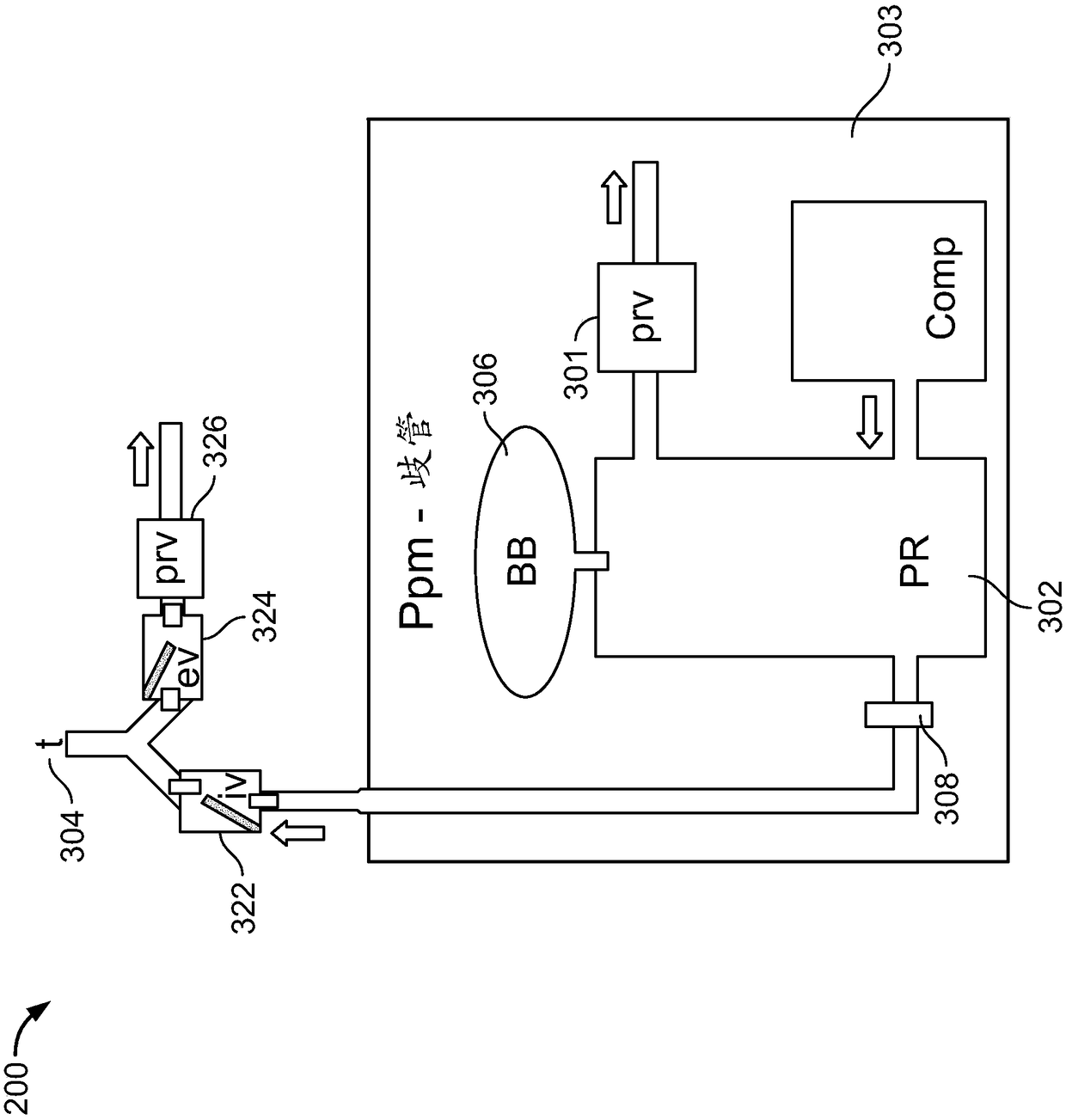
Regeneration of a functional pulmonary vascular bed
A pro-angiogenic, vascular technology for the regeneration of functional pulmonary vascular beds that can address issues that limit long-term outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0128] The following specific examples further illustrate the present invention.
example 1
[0130] Study of the Vascular Bed of the Acellular Pulmonary
[0131] The purpose of this example was to characterize the perfusion properties of the acellular vascular bed in a whole lung scaffold using an in vitro microsphere perfusion assay. By quantifying microspheres harvested from the PV, trachea, and lung periphery, we demonstrate both the continuity and integrity of the vascular basement membrane after decellularization and thereby confirm compartment-specific cell delivery in acellular lung scaffolds possibility. Microsphere quantification further implicates fluid-driven hydrostatic pressure loss rather than particle leakage as the primary cause of low passage efficiency. By applying these findings to recellularization, efficient and uniform endothelial coverage is achieved through combined arterial and venous cell delivery.
[0132] method
[0133] Cadaveric lungs were removed from male Sprague-Dawley rats (250-300 g, Charles River Laboratories) following systemic ...
example 2
[0143] Improved endothelial delivery
[0144] The purpose of this example is the reconstitution of viable endothelial cells on cell-free rat lung scaffolds and confirmation of the integrity of the vascular basement membrane after decellularization.
[0145] method
[0146] Cell seeding into decellularized rat lungs was performed in bioreactors similar to those described herein, allowing cell delivery and perfusion from PA and PV. The trachea was cannulated and opened to the interior of the bioreactor through a port located approximately 5 cm above the PA level. Decellularized lung scaffolds were filled by perfusing with 100 ml of Hank's balanced salt solution containing human fibronectin (2.5 μg / ml) at 1 ml / min for 1 hour, washed with Hank's balanced salt solution for 1 hour, and Equilibrate in the respective media for at least 1 h before cell seeding. For endothelial delivery by PA, 40 million HUVECs were resuspended in a single inoculation chamber with 100 ml EGM-2 and in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com