rhTNK-tPA lyophilized preparation for injection and preparation method of rhTNK-tPA lyophilized preparation for injection
A technology for freeze-dried preparations and injections, applied in the field of biopharmaceuticals, can solve the problems of low activity recovery rate of target protein, achieve high purity and recovery rate, good appearance, and avoid stratification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
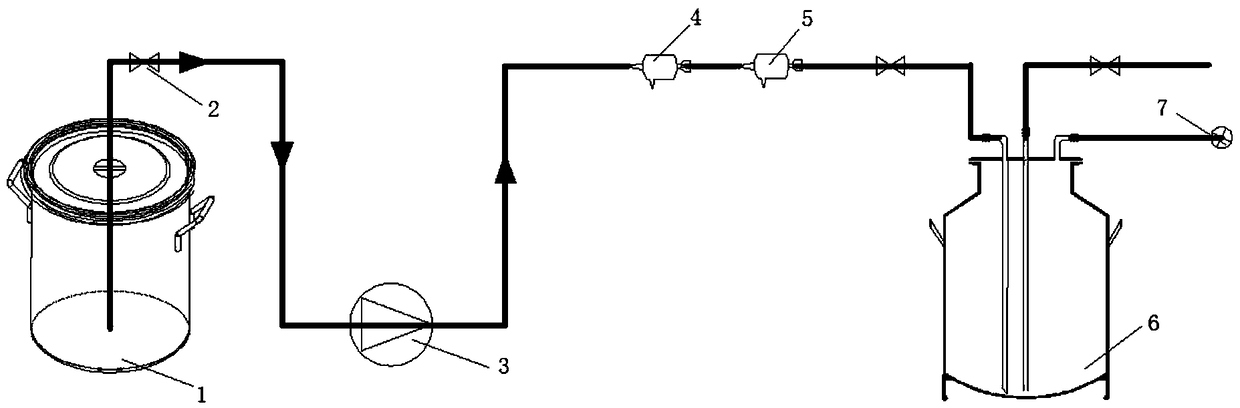
Image
Examples
Embodiment 1
[0035] Example 1, a rhTNK-tPA freeze-dried preparation for injection and its preparation method
[0036] A) separation and purification:
[0037] S1, Blue Sepharose 6FastFlow affinity chromatography: before performing chromatography, the chromatography column is equilibrated with 2.5 times column volume buffer ①, and its components are 20mM phosphate buffer and 0.04% polysorbate 80, pH7.2; for sample loading, use 2.5 times the column volume of buffer ② to wash out the impurity protein, the components are: 20mM phosphate buffer, 2M sodium chloride and 0.04% polysorbate 80, pH7.2; use 3 times The target protein was obtained by eluting with the column volume of eluent I. The components of eluent I were 20mM phosphate buffer, 1M sodium chloride, 2M urea and 0.04% polysorbate 80, and the pH value of the eluent was 7.0;
[0038] S2. Lysine HyperD affinity column chromatography: The equilibration and sample loading process is the same as that of the B column above. After completion,...
Embodiment 2
[0055] Example 2, a rhTNK-tPA freeze-dried preparation for injection and its preparation method
[0056] The difference between Example 2 and Example 1 is that the separation and purification steps become: through Blue Sepharose 6FastFlow affinity chromatography, Sephadex G-25 gel filtration chromatography, lysine affinity chromatography and Capto Q anion chromatography to obtain For the purified rhTNK-tPA, other parameters are the same as in Example 1.
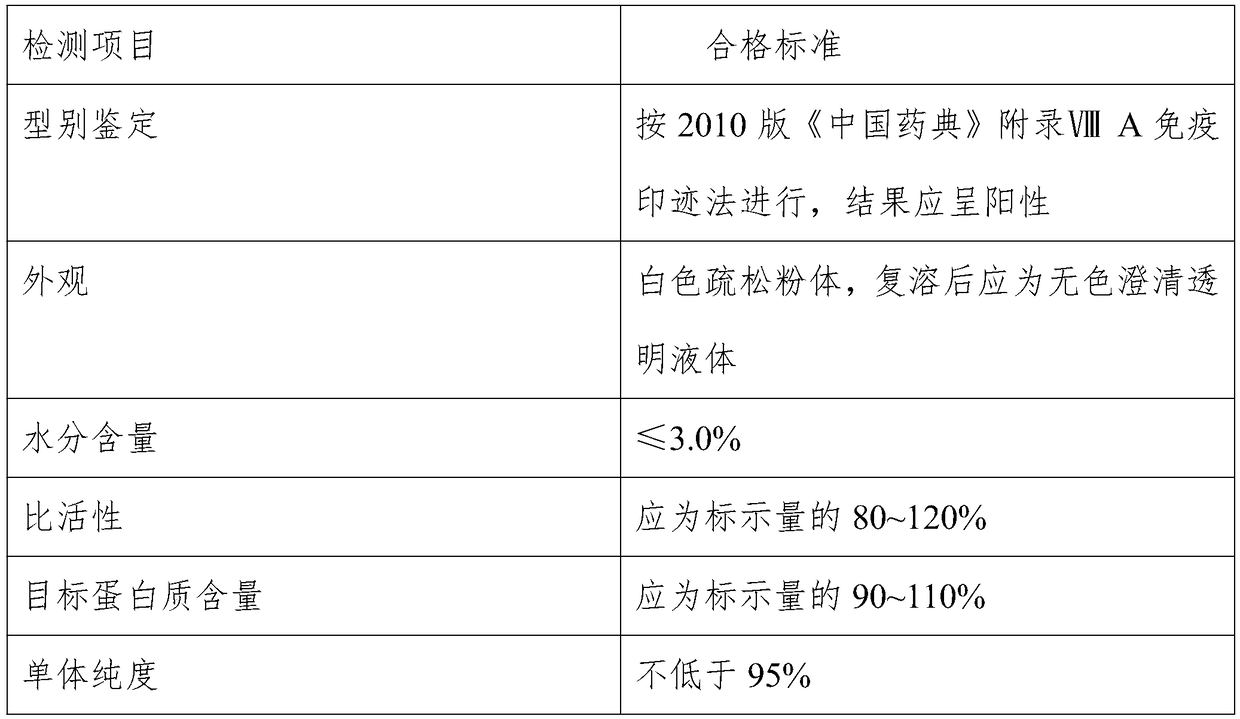
[0057] After purification, the activity recovery rate of the target protein was detected, and the activity recovery rate of rhTNK-tPA was 96.2%.
Embodiment 3
[0058] Example 3, a rhTNK-tPA freeze-dried preparation for injection and its preparation method
[0059] The difference between embodiment 3 and embodiment 1 is that in the pre-freezing stage, the step of pre-freezing at -15°C is removed, and other parameters are the same as in embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com