MOFs (Metal Organic Frameworks)-based NiCo@N-C dual-function oxygen electrode catalyst and preparation method thereof
A technology of oxygen electrode and catalyst, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effects of large specific surface area, increased N doping amount and conductivity, and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of NiCo(1:1)@N-C bifunctional oxygen electrode catalyst:
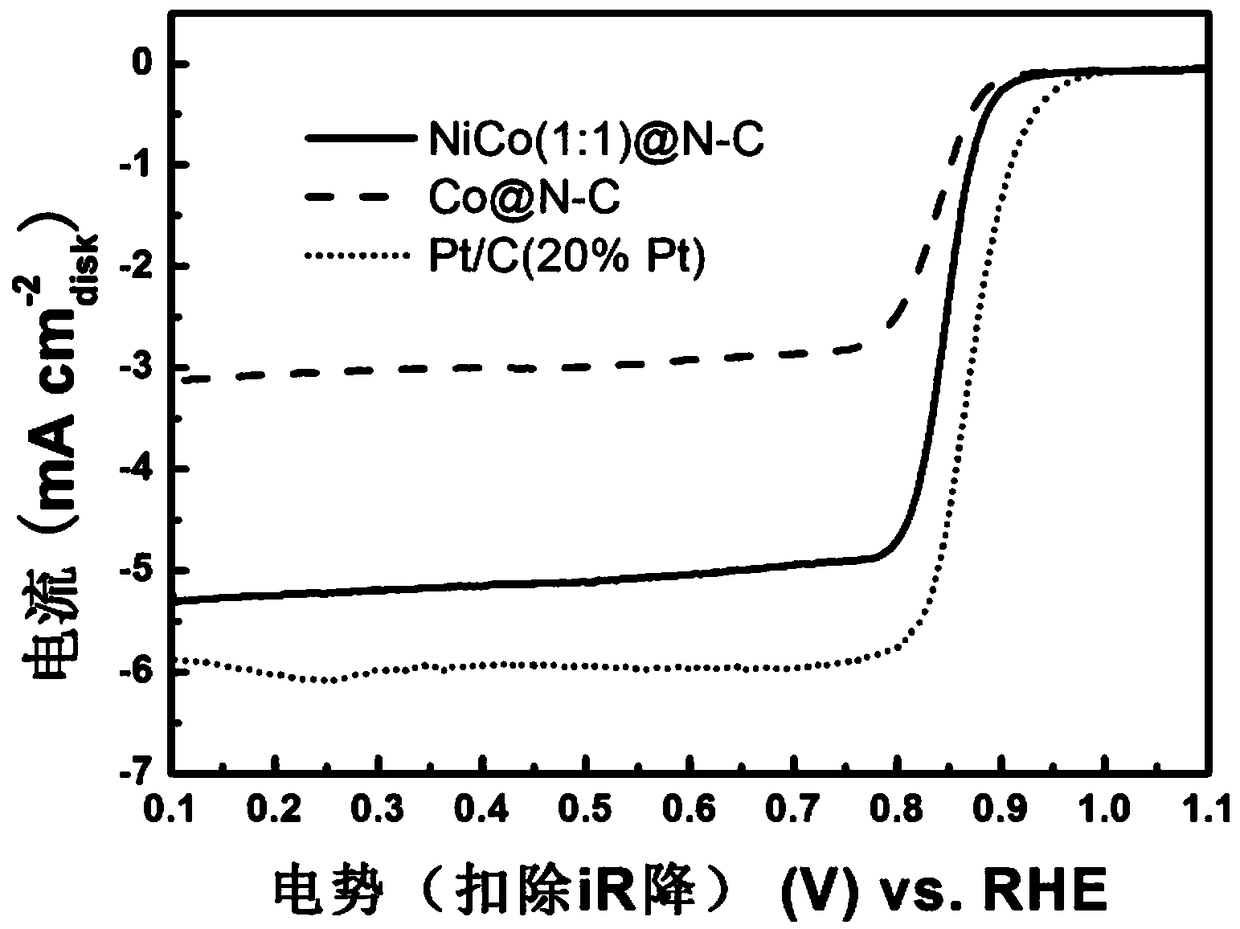
[0039] Weigh 436.1mg Ni(NO 3 ) 2 .6H 2 O and 436.6mg Co(NO 3 ) 2 .6H 2 O was dissolved in methanol with a volume of 30mL and mixed evenly by ultrasonic to make metal precursor solution A; in addition, 985.2mg MeIM was weighed and dissolved in 10mL methanol and mixed evenly to make organic ligand solution B; the metal ion Ni 2+ with Co 2+ The total molar concentration in the organic reagent is 0.075mol / L; under the condition of magnetic stirring, the organic ligand solution B is gradually added to the metal precursor solution A to form a mixed solution, and the stirring is continued for 1h; In a hydrothermal kettle with a tetrafluoroethylene liner (50mL, filling degree 80%), conduct a solvothermal reaction at 100°C for 12 hours; vacuum filter the mixture obtained from the solvothermal reaction, wash it with methanol several times, and dry it in vacuum at 80°C Take it out after 12h to obtain a p...
Embodiment 2
[0044] With reference to the preparation method and test method of embodiment 1 catalyst, difference is that n(Ni 2+ ):n(Co 2+ ) adjusted to 1:2, namely weigh 581.6mg Ni (NO 3 ) 2 .6H 2 O and 1164.1mg Co(NO 3 ) 2 .6H 2 O was dissolved in methanol with a volume of 40mL, and ultrasonically mixed uniformly to make metal precursor solution A; in addition, 1.97g MeIM was weighed and dissolved in 40mL methanol and mixed uniformly to make organic ligand solution B; the metal ion Ni 2+ with Co 2+ The total molar concentration in the organic reagent is 0.075mol / L; NiCo(1:2)@N-C bifunctional oxygen electrode catalyst is prepared.
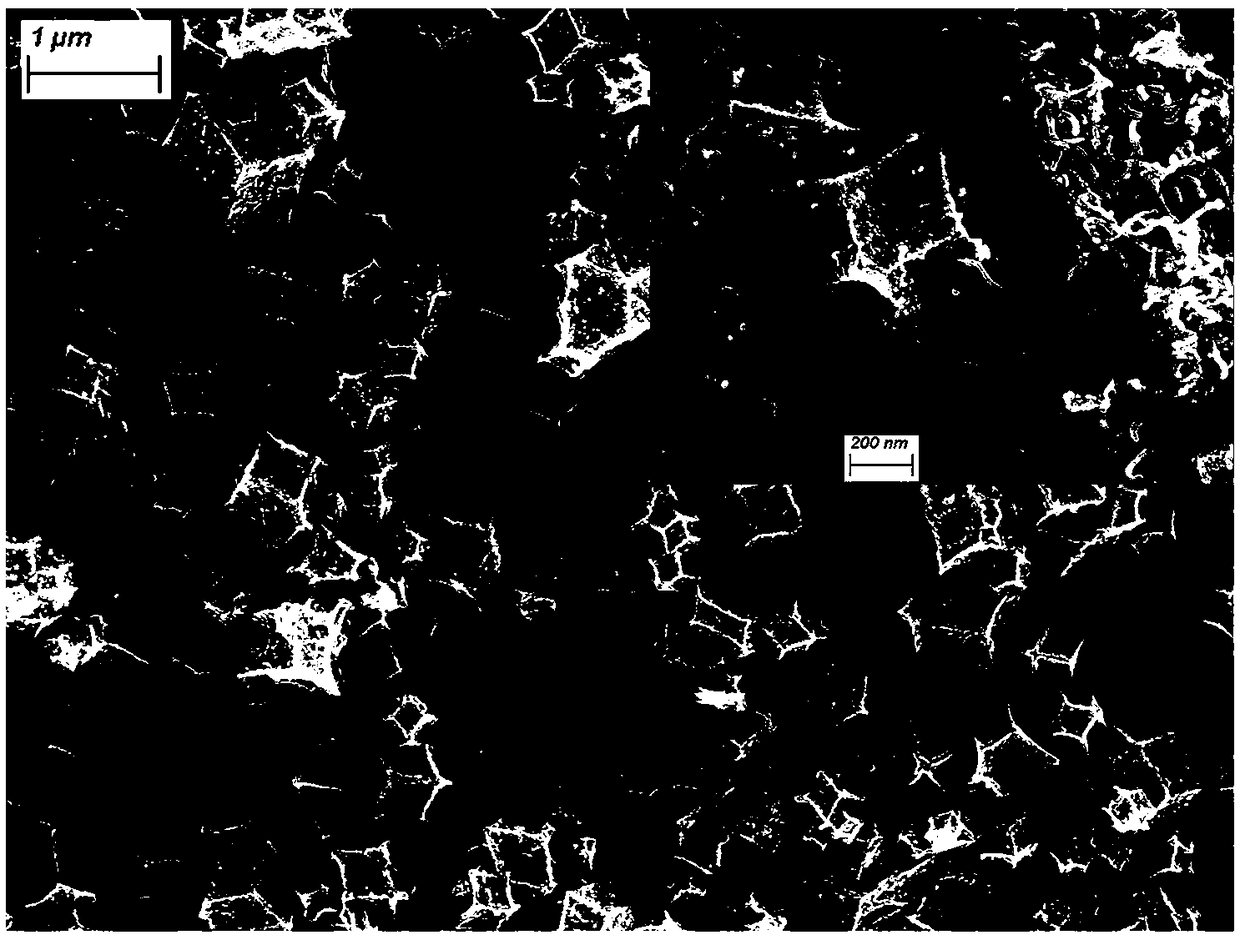
[0045] The NiCo(1:2)@N-C bifunctional oxygen electrode catalyst prepared in FE-SEM test embodiment 2, such as Figure 5 As shown, the obtained material is a rhombic dodecahedron with a slightly collapsed surface, and there are a small amount of carbon nanotube structures on each surface except spherical protrusions. The surface area of the catalyst...
Embodiment 3
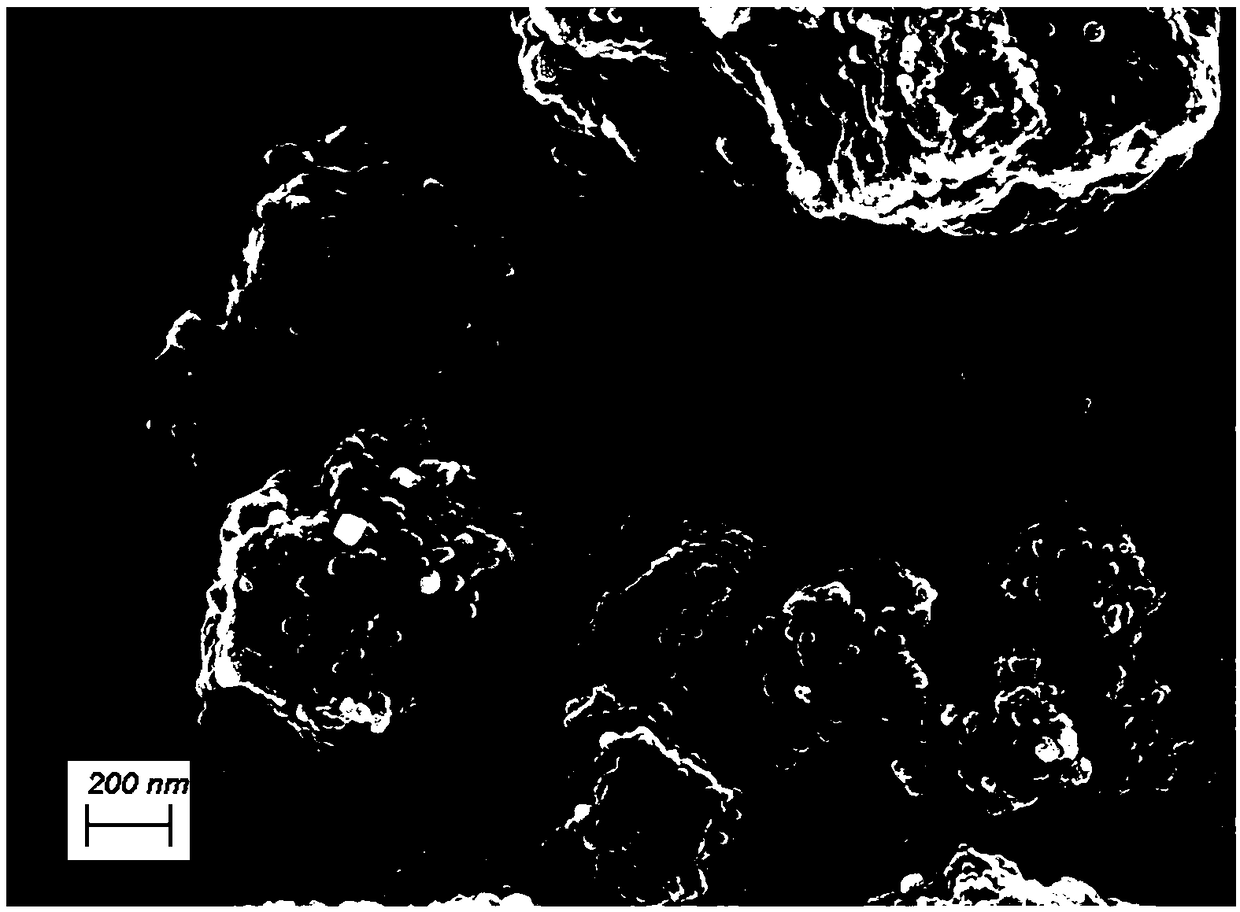
[0047] With reference to the preparation method and test method of embodiment 1 catalyst, difference is during liquid preparation, n(Ni 2+ ):n(Co 2+ ) adjusted to 1:4, that is, weigh 174.47mg Ni (NO 3 ) 2 .6H 2 O and 698.47mg Co(NO 3 ) 2 .6H 2 O was dissolved in methanol with a volume of 20mL, and ultrasonically mixed evenly to make metal precursor solution A; in addition, 985.2mg MeIM was weighed and dissolved in 10mL methanol and mixed evenly to make organic ligand solution B; the metal ion Ni 2+ with Co 2+ The total molar concentration in the organic reagent is 0.10mol / L; NiCo(1:4)@N-C bifunctional oxygen electrode catalyst is prepared. The surface area of the catalyst obtained in the embodiment 3 tested by BET method is 265.7 m 2 / g, the total pore volume is 0.20cm 3 / g. The atomic ratio of C:N:O:Co:Ni on the surface of the catalyst obtained in Example 3 by XPS test is 83.90:3.87:10.1:1.61:0.52, combined with XRD analysis, it can be known that the catalyst is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com