Supported type high-selectivity bimetallic catalyst with core-shell structure as well as preparation method and application thereof
A bimetallic catalyst, high selectivity technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, catalyst, etc., can solve the problem of low propylene selectivity of pure Pt catalyst, and achieve good results , reduced dosage, high propylene selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
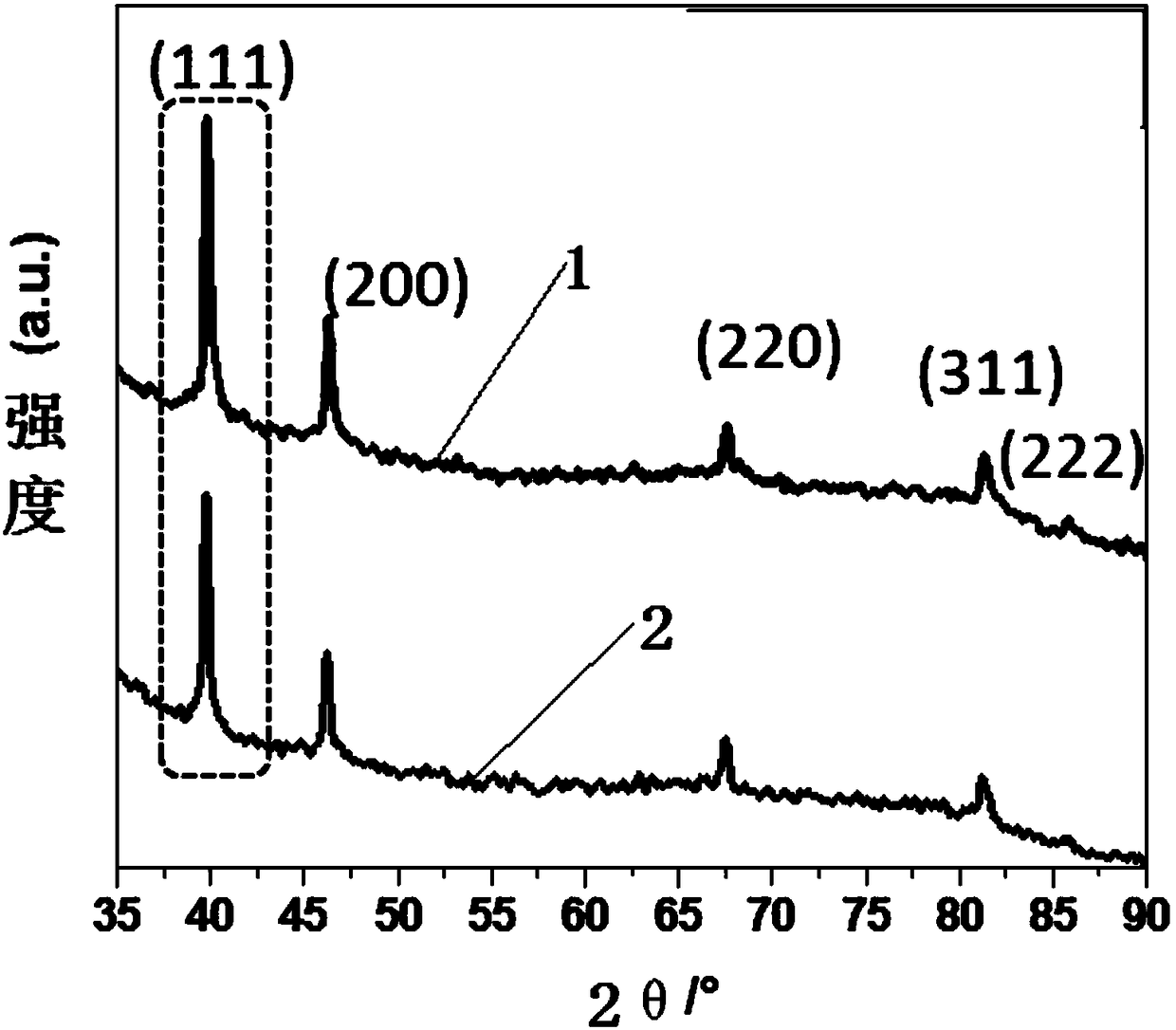
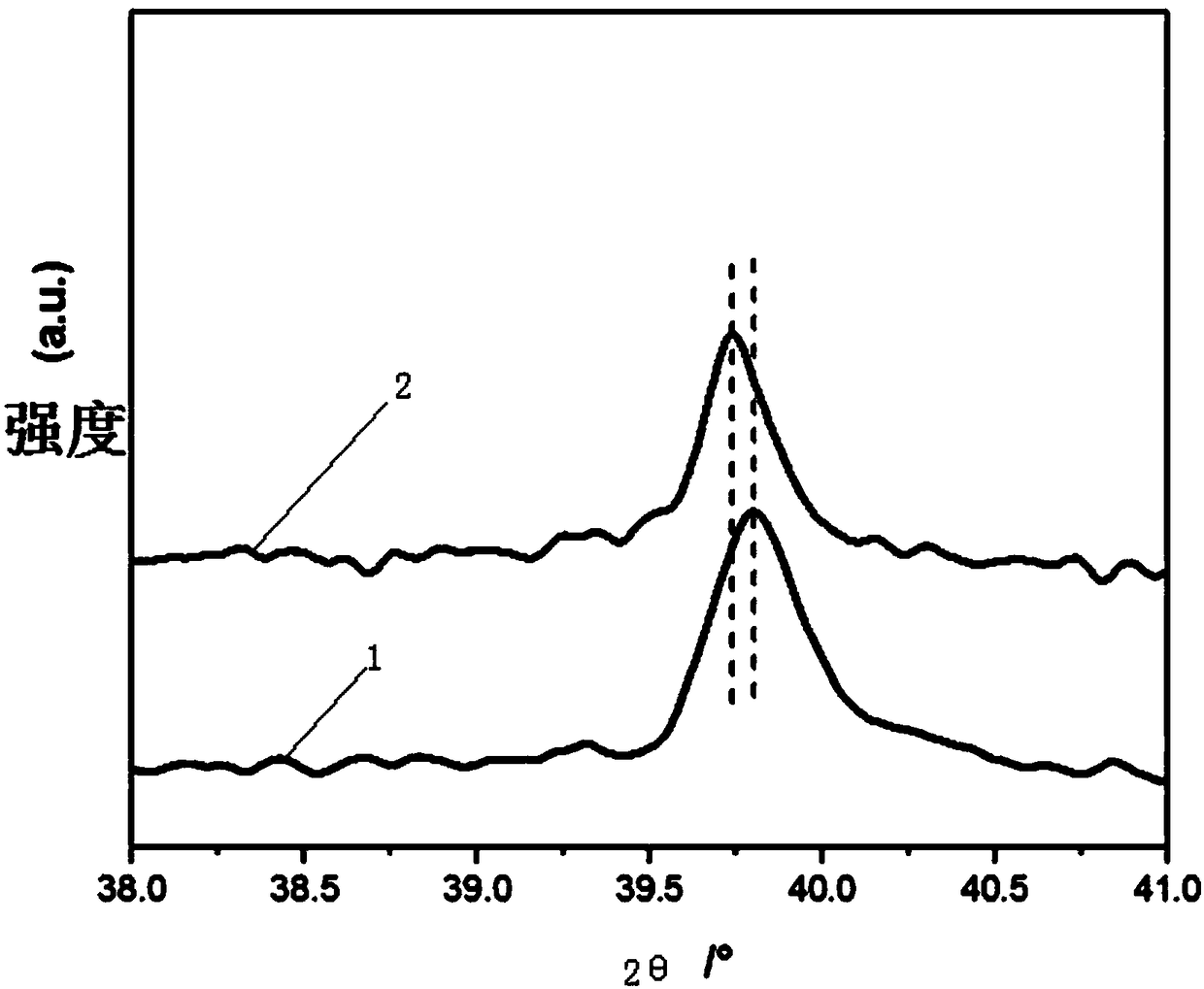
Image
Examples
Embodiment 1
[0038] (1) Take 5ml of deionized water and 6ml of ethanol in a 100ml beaker and stir until evenly mixed, add 0.75ml of 0.010g / ml chloroplatinic acid solution prepared in advance, and nitrate of transition metal Fe prepared in advance Solution, so that Pt:Fe=3:1 (molar ratio), continue to stir. At this point a weighed 1 g of SBA-15 was added to the stirred solution.
[0039] (2) After stirring for 24 hours, the beaker was dried in an oven at 80° C. for 12 hours when the solution became gelatinous.
[0040] (3) Grind the dried solid into powder, put it in a crucible and bake it in a muffle furnace at 300° C. for 2 h (at a heating rate of 2° C. / min).
[0041] (4) the catalyst that roasting is finished is placed in the quartz boat of high temperature resistance, and is placed in tube furnace, feeds 5% H 2 / Ar, reduced at 400°C for 4h (heating rate 2°C / min).
[0042] (5) Get 0.3 g of the reduced catalyst and put it in the dilute nitric acid solution of 0.0005 mol / l, vibrate ultr...
Embodiment 2
[0055] Adopt the method of Example 1 to react, the only difference is that step (5) the resting time after the catalyst in the nitric acid solution is oscillated and ultrasonicated is 5min, and the obtained catalyst is PtFe@Pt / SBA-15 that has been pickled for 5min--- 5min catalyst.
Embodiment 3
[0057] Adopt the method of Example 1 to react, the only difference is that step (5) the resting time after the catalyst in the nitric acid solution is oscillated and ultrasonicated is 20min, and the obtained catalyst is PtFe@Pt / SBA-15 that has been pickled for 20min--- 20min catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com