Propylene tethered ciprofloxacin-isatin hybrids as well as synthetic method and application thereof
A technology of indolindione and ciprofloxacin, which is applied in the field of medicinal chemistry and can solve the problems of poor therapeutic effect on bacterial infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
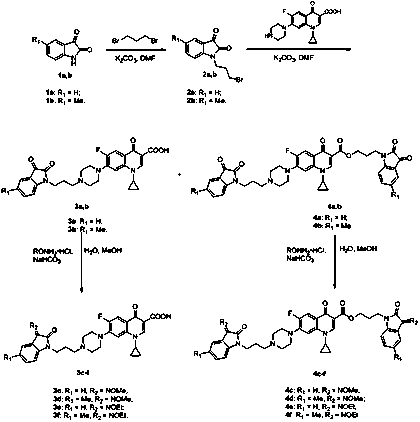
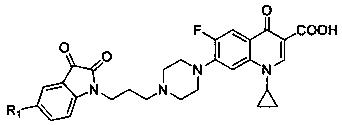
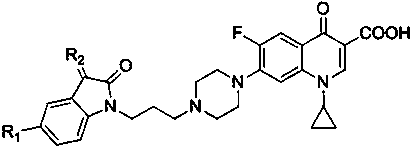
[0029] figure 1 The synthesis routes of propylene-tethered indolindione-ciprofloxacin hybrids 3a-f and 4a-f are given. Alkylation of C-5-substituted indolindione 1a,b with 1,3-dibromopropane in the presence of potassium carbonate to generate N-(3-bromopropyl)indolindione 2a ,b, and then these intermediate products are incorporated into the CPFX core, and then the expected target products 3a,b and bis-indolindione-ciprofloxacin hybrids 4a,b can be obtained. Subsequently, the hybrids 3a,b or 4a,b were condensed with methylhydroxylamine or ethylhydroxylamine hydrochloride in the presence of sodium bicarbonate to generate other conjugates 3c-f and 4c-f.
[0030] In this study, all propylene-tethered indolindione-ciprofloxacin hybrids 3a-f and 4a-f were tested for their in vitro antibacterial activity against clinically typical Gram-positive and Gram-negative bacteria. evaluation, and also on the MTB H 37 The antimycobacterial activity of Rv and MDR-TB strains was evaluated. Am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com