Concise glufosinate ammonium synthesizing method
A synthesis method and glufosinate-ammonium technology are applied in the chemical industry to achieve the effects of simplified operation steps, good application prospects and reduced labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
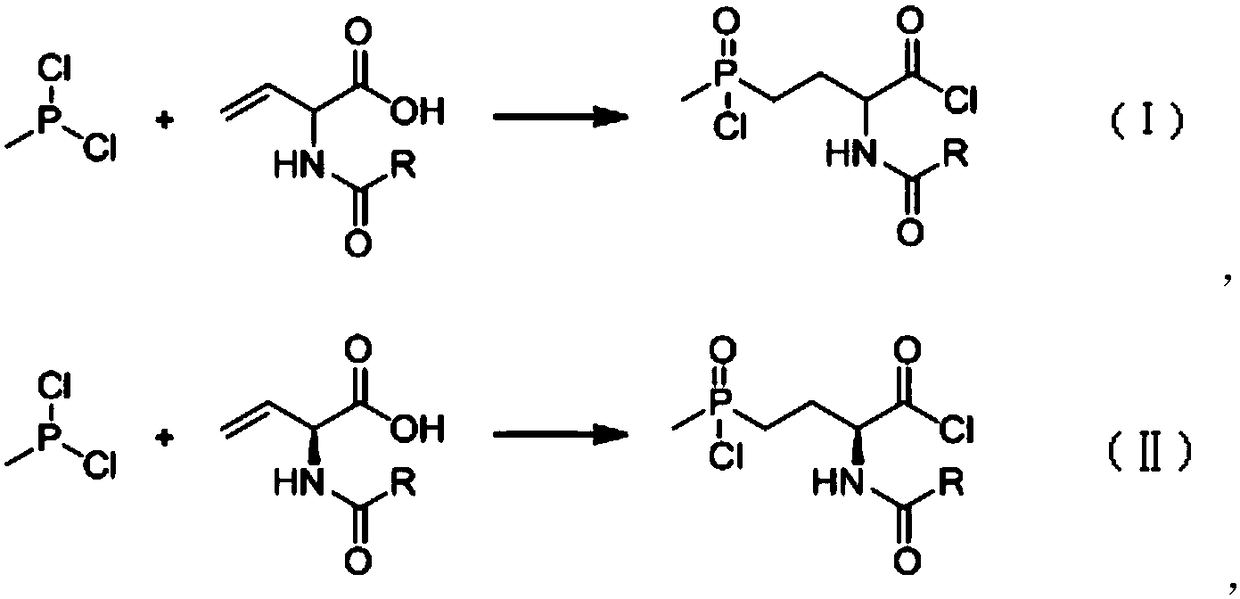
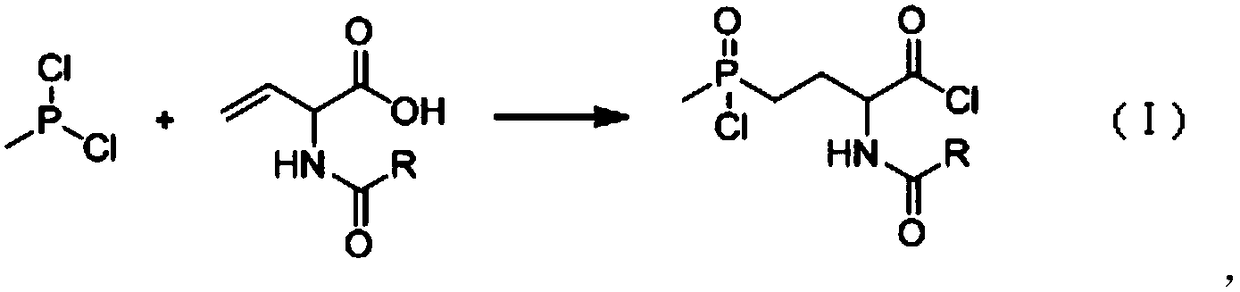
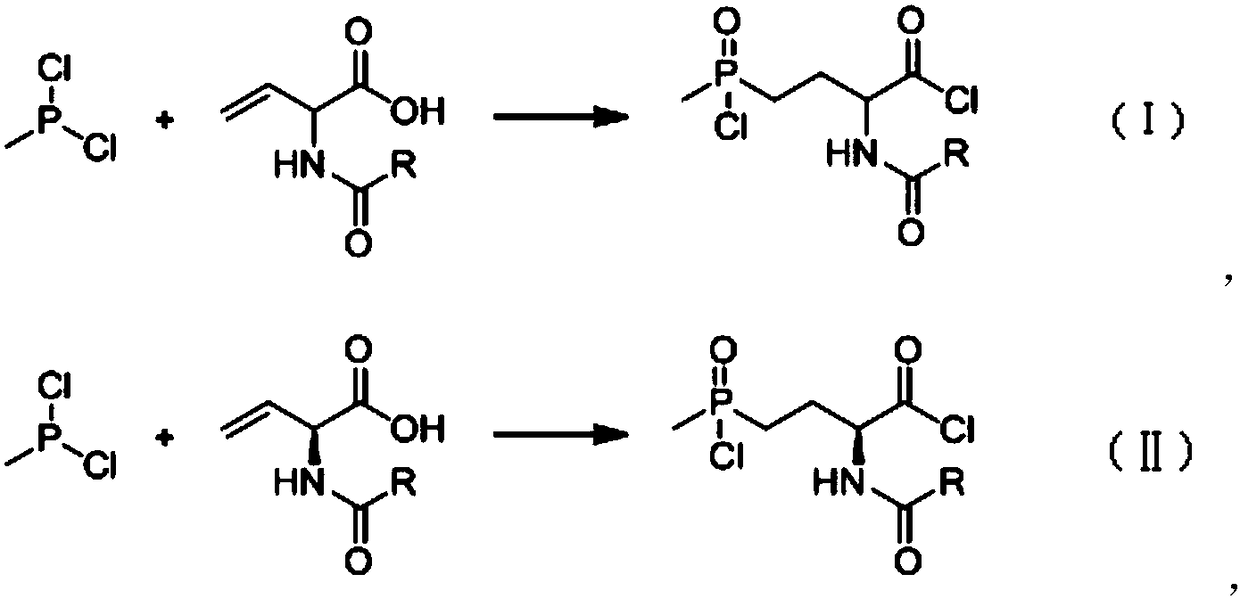
[0043] 90.67kg (686.5mol) of N-acetyl-2-aminobutenoic acid (97% in absolute content) was dissolved in 200 liters of methyl tetrahydrofuran to obtain the first solution; then the first solution was added dropwise to the methyl In 81.9kg of phosphine dichloride (98%, obtained by rectification), the temperature is controlled within 70-90°C. After the dropwise addition is completed, the reaction solution is kept at 85-90°C for 1 hour, and the sample is tested to prove that the reaction has been completed. , to obtain the second reaction solution; while hot, the second reaction solution is put into another 1000 liter reaction kettle, the temperature is controlled in the range of 0 to 30°C, and under stirring conditions, slowly add 38.2 kilograms of water dropwise, after the dropwise addition is completed, then Add 60 kg of water, then heat up to reflux for 3 to 4 hours to detect that the amide has been hydrolyzed, then slowly add 365 kg of 30% sodium hydroxide to obtain the third re...
Embodiment 2
[0045] Dissolve 90.67kg (686.5mol) of (2S)-N-acetyl-2-aminobutenoic acid (97% in absolute content) in 200 liters of methyl tetrahydrofuran to obtain the first solution; then drop the first solution Add it to 81.9kg of methylphosphine dichloride (98%, obtained by rectification), and control the temperature within 70-90°C. After the dropwise addition, keep the reaction solution at 85-90°C for 1 hour, and take a sample for detection. It is proved that the reaction has been completed, and the second reaction solution is obtained; the second reaction solution obtained is put into another 1000-liter reaction kettle while it is hot, and the temperature is controlled within the range of 0-30°C. Under the condition of stirring, slowly add 38.2 kg of water, dropwise After completion, add 60 kilograms of water again, then heat up to reflux reaction for 3 to 4 hours, detect that the amide has been hydrolyzed, slowly add 365 kilograms of 30% sodium hydroxide to obtain the third reaction sol...
Embodiment 3
[0047] 90.67kg (686.5mol) of N-acetyl-2-aminobutenoic acid (97% in absolute content) was dissolved in 200 liters of methyl tetrahydrofuran to obtain the first solution; then the first solution was added dropwise to the methyl In phosphine dichloride 90kg (98%, rectification gained), temperature is controlled within 65~80 ℃, after dropwise addition is completed, the reaction solution is incubated and reacted for 1 hour at 75~80 ℃, and sampling inspection proves that the reaction has been completed. Obtain the second reaction liquid; put the obtained second reaction liquid into another 1000-liter reaction kettle while it is hot, control the temperature in the range of 0-10°C, and slowly add 38.2 kg of water dropwise under stirring conditions. After the dropwise addition, add 60 kg of water, then heated up to reflux reaction for about 3 hours, detected that the amide had been hydrolyzed, cooled to normal temperature (20-30°C), controlled the temperature at 20°C-30°C, slowly added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com