Preparation method of germacrone oxobutyric acid
A technology of gemmanone oxobutyric acid and gemmanone, which is applied in the field of preparation of gemmanone oxobutyric acid, can solve problems such as no preparation method reported in literature, difficulty in structural modification, and not being particularly stable, and achieves low cost , easy structural modification and transformation, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
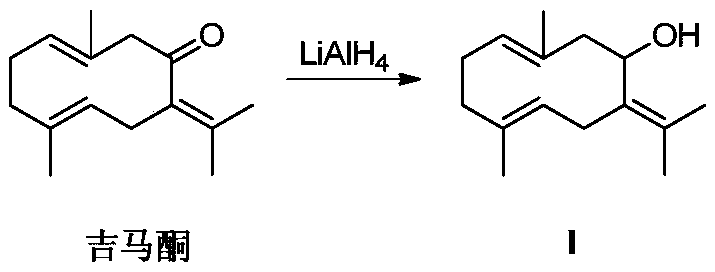
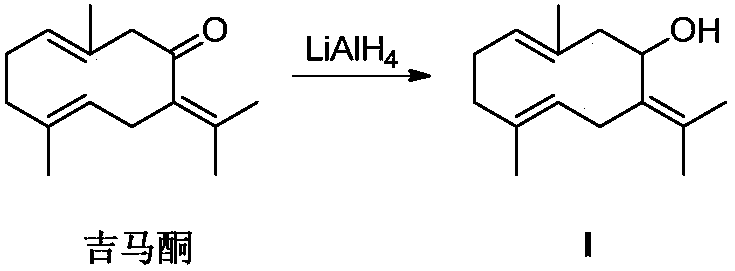
[0015] Embodiment 1: the synthesis of gemmacone reduction product (compound I)
[0016]
[0017] Nitrogen protection, the LiAlH 4 (70mg, 1.835mmol) was dissolved in anhydrous tetrahydrofuran, cooled to -15°C, under vigorous stirring, slowly added dropwise a tetrahydrofuran solution of gemmaconone (100mg, 0.459mmol), stirred for 10min, and reacted in an ice bath for 2h (monitored by TLC). Add 15% sodium hydroxide solution and anhydrous sodium sulfate, stir for 5 minutes, filter, wash the filter cake with dichloromethane, and spin the filtrate to obtain a crude product, which is separated and purified by column chromatography (PE:EA=10:1) to obtain Compound I, a colorless oil. NMR data ( 1 HNMR, 400MHz, internal standard TMS, solvent DMSO-d6) as follows: δppm 4.67 (s, 1H, OH), 4.58 (m, 1H, CH), 3.10 (q, J=10.4Hz, 1H, CH), 2.22 ( m,3H,CH),2.08-1.67(m,6H,CH 2 ),1.60(s,6H,CH 3 ),1.45(s,6H,CH 3 ).
Embodiment 2
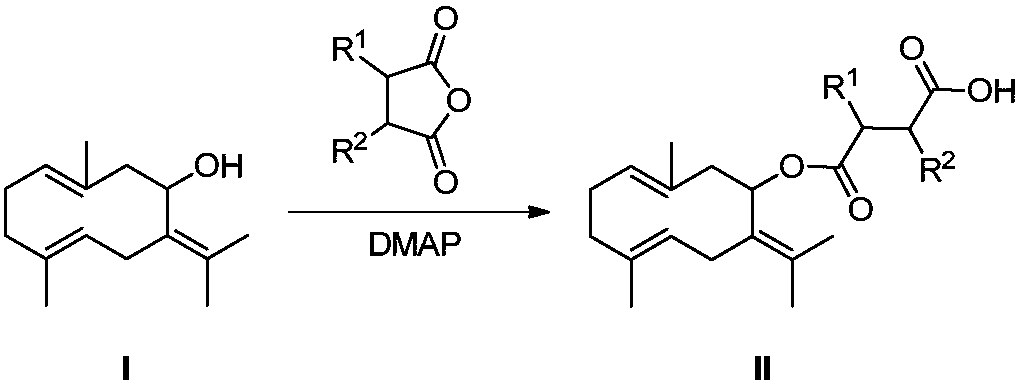
[0018] Embodiment 2: the synthesis of gemmaconone oxobutanoic acid (compound II)
[0019]
[0020] Nitrogen protection, in a 100mL single-necked flask, add gemmaconone reduction product (compound I, 120mg, 0.545mmol), succinic anhydride (54.6mg, 0.545mmol), DMAP (80mg, 0.655mmol) and 10mL of anhydrous tetrahydrofuran, 75 °C and stirred overnight. After filtration, the filtrate was spin-dried, separated and purified by column chromatography (PE: EA = 1: 1) to obtain compound II as a colorless oil. NMR data ( 1 HNMR, 400MHz, internal standard TMS, solvent DMSO-d6) as follows: CH),2.72-2.63(m,4H,CH 2 ), 2.28 (d, J=11.6Hz, 2H, CH 2 ),2.16-2.08(m,6H,CH 2 ),1.84(s,3H,CH 3 ),1.76(s,3H,CH 3 ),1.74(s,3H,CH 3 ),1.68(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com