Drug unified coding rule management method and code identifier taking medical insurance payment as core
A management method and drug technology, applied in the field of information processing, can solve problems such as ununiform coding of business departments and systems, lack of coding rules, traceability, etc., to achieve the effect of promoting sharing and communication, and facilitating mutual transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
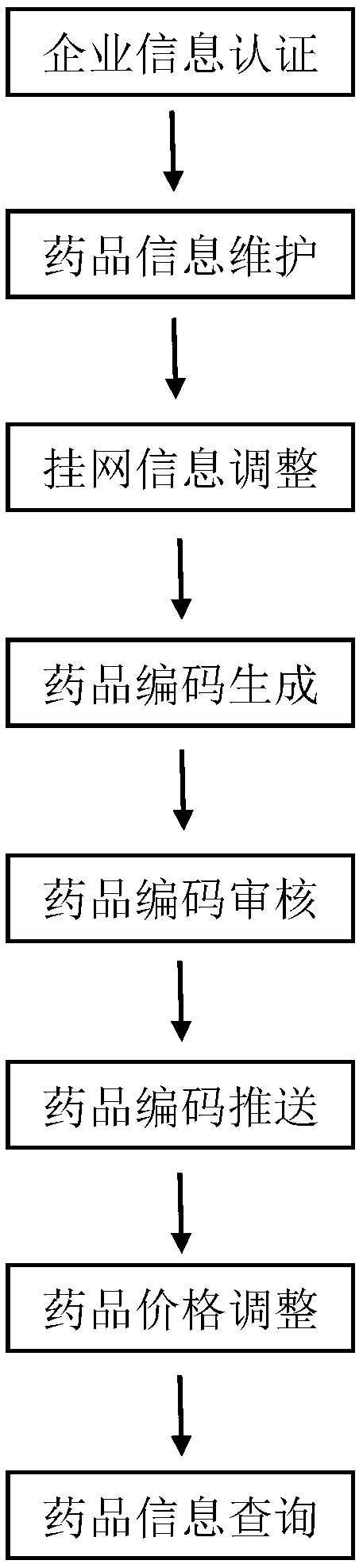
[0054] Such as figure 1 As shown, a unified drug coding rule, management method and code recognizer with medical insurance payment as the core, including the following steps,
[0055] Step 1, enterprise information certification: drug production enterprises submit valid qualifications, and the drug trading platform conducts key qualification certification. Pharmaceutical production enterprises that meet the qualification requirements obtain a unique 6-digit enterprise code, and can maintain enterprise information and drug information through the platform.
[0056] Step 2, drug information maintenance: drug manufacturers maintain enterprise information and drug information through the drug trading platform, and provide necessary qualification support for the maintained information.
[0057] Step 3: Adjustment of listing information: The drug trading platform conducts certification according to the drug information provided by the drug manufacturer, confirms whether it meets th...
Embodiment 2
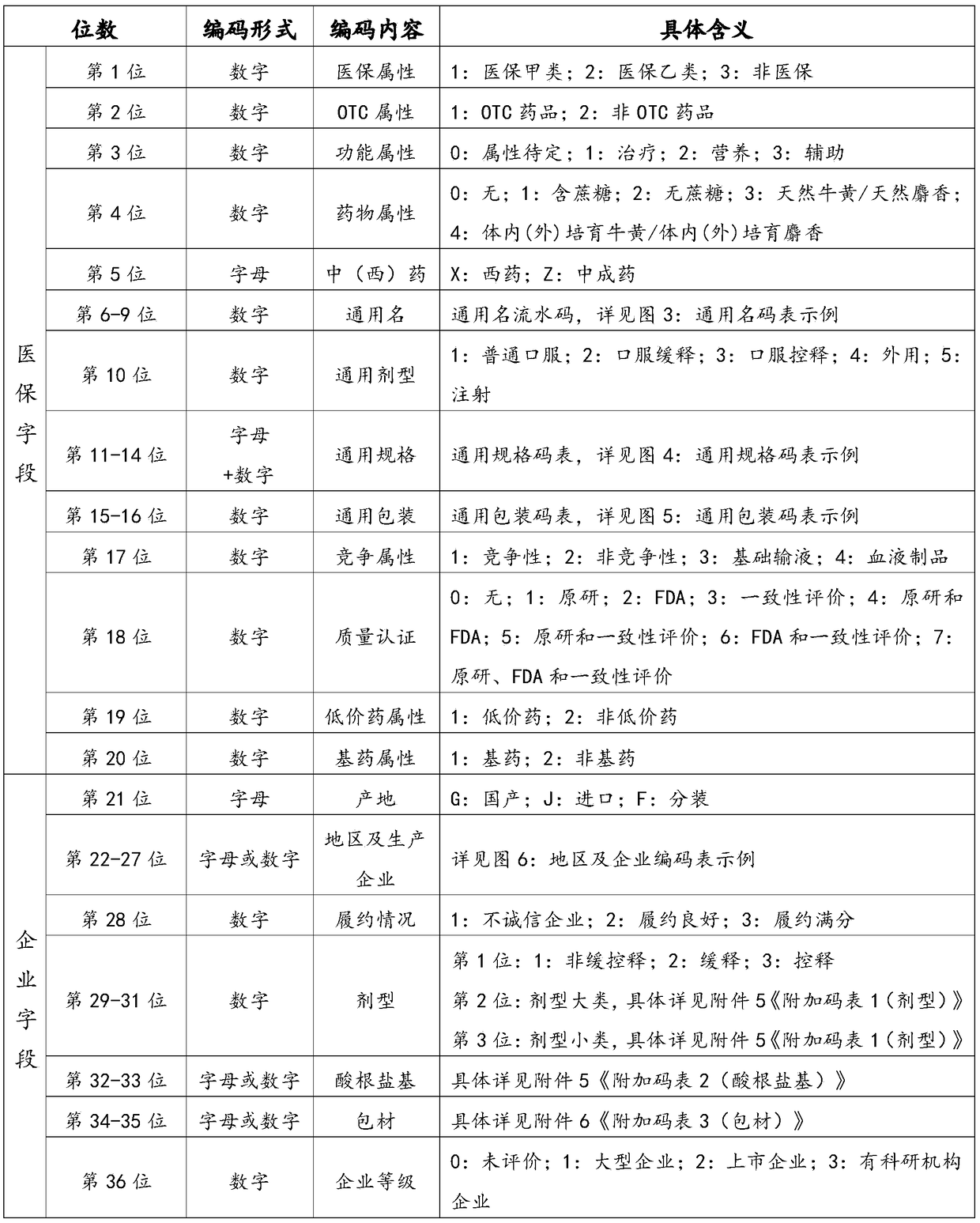
[0065] Such as figure 2 As shown, a kind of Chinese medicine (Zhibitai Capsule) is selected to encode, and the encoding is:
[0066] 1-2-1-Z0100-1-A1A8-10-1-0-1-0-G-510205-3-021-01-01-0
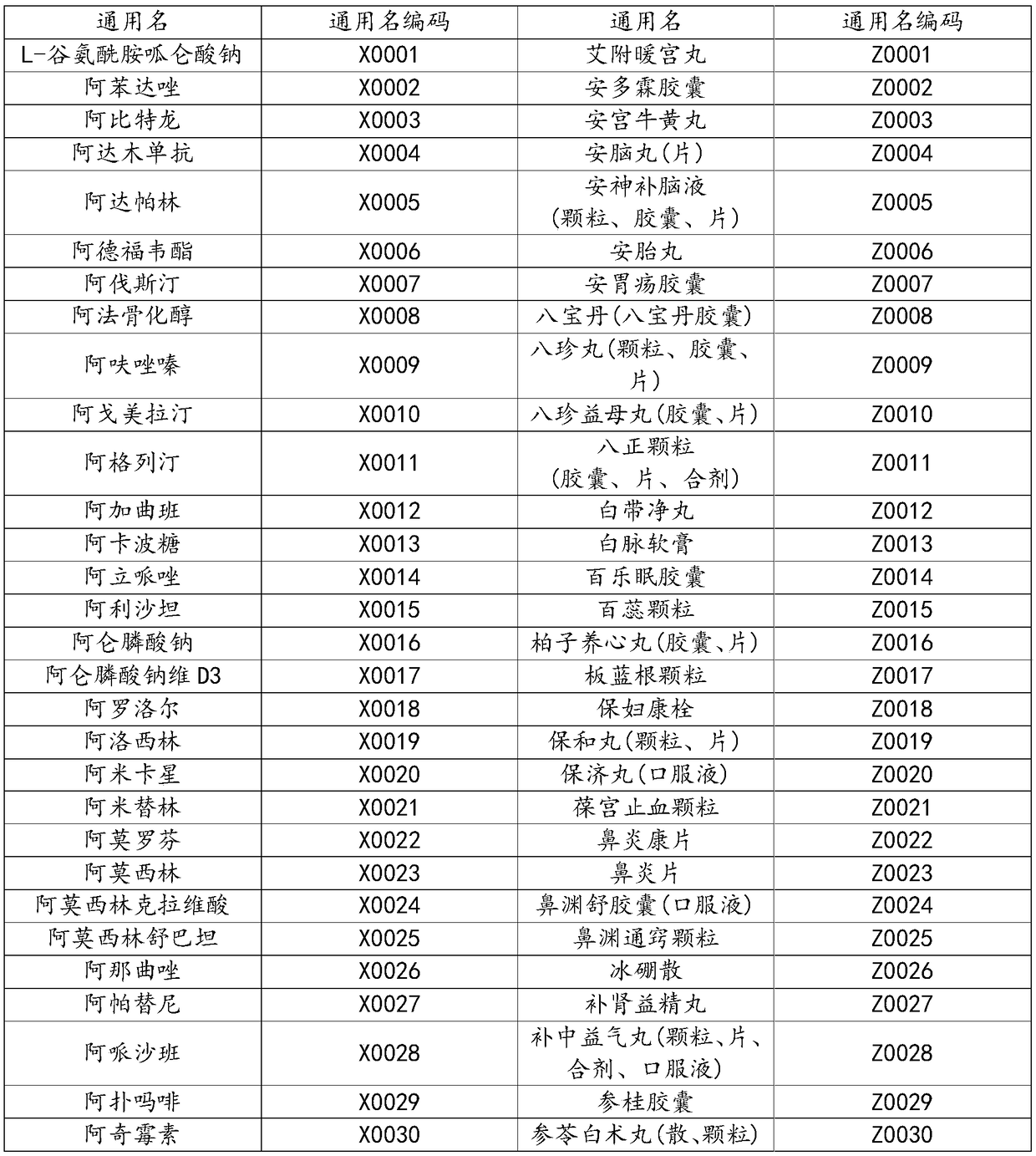
[0067] Among them, 1-2-1 represents medical insurance, OTC attribute RX, treatment; Z0100 represents the common name of traditional Chinese medicine, oral preparation of compound red yeast rice; 1-A1A8-10 represents the general dosage form is oral, the general specification is 0.24g, and the general packaging is 10 Tablets / box; 1-0-1-0 represents competition, no special certification, low-priced drugs, non-essential drugs; G-510205 represents domestic production, Chengdu Di’ao Jiuhong Pharmaceutical Factory, full marks for contract performance; 3-021-01- 01-0, representing non-sustained and controlled release, capsule, hydrochloride, packaging material is 01 attribute, no special drug attribute.
Embodiment 3
[0069] Such as figure 2 Shown, choose a kind of western medicine (roxithromycin sheet) to encode, and be encoded as:
[0070] 1-2-1-X0709-1-A792-10-1-0-1-0-G-311619-3-011-00-01-0
[0071] Among them, 1-2-1 represents medical insurance, OTC attribute RX, treatment; X0709 represents the generic name of western medicine, roxithromycin; 1-A792-10 represents the general dosage form is oral, the general specification is 50mg, and the general packaging is 10 tablets / Box; 1-0-1-0 represents competition, no special certification, low-priced drugs, non-essential drugs; G-311619 represents domestic production, Shanghai Modern Pharmaceutical Co., Ltd., full marks for performance; 3-011-00-01-0 , stands for non-sustained and controlled release, tablet / film-coated tablet, no acid radical, packaging material is 01 attribute, no special drug attribute.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com