Novel compound and medical compositions for treating tuberculosis comprising the same
A medicinal composition and a technology for tuberculosis, which are applied in the field of compositions for the prevention or treatment of tuberculosis, and can solve problems such as the lack of replacement in effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
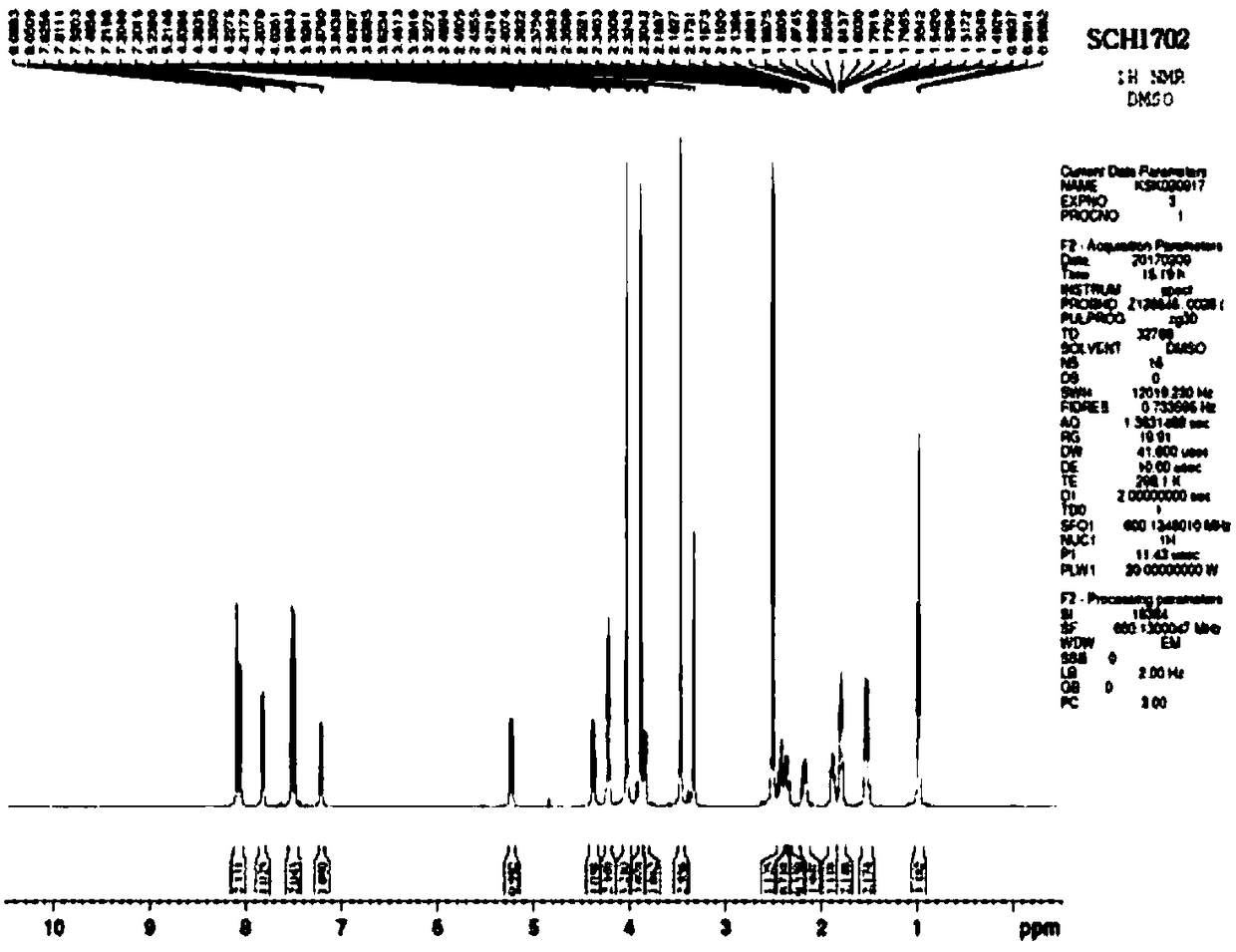
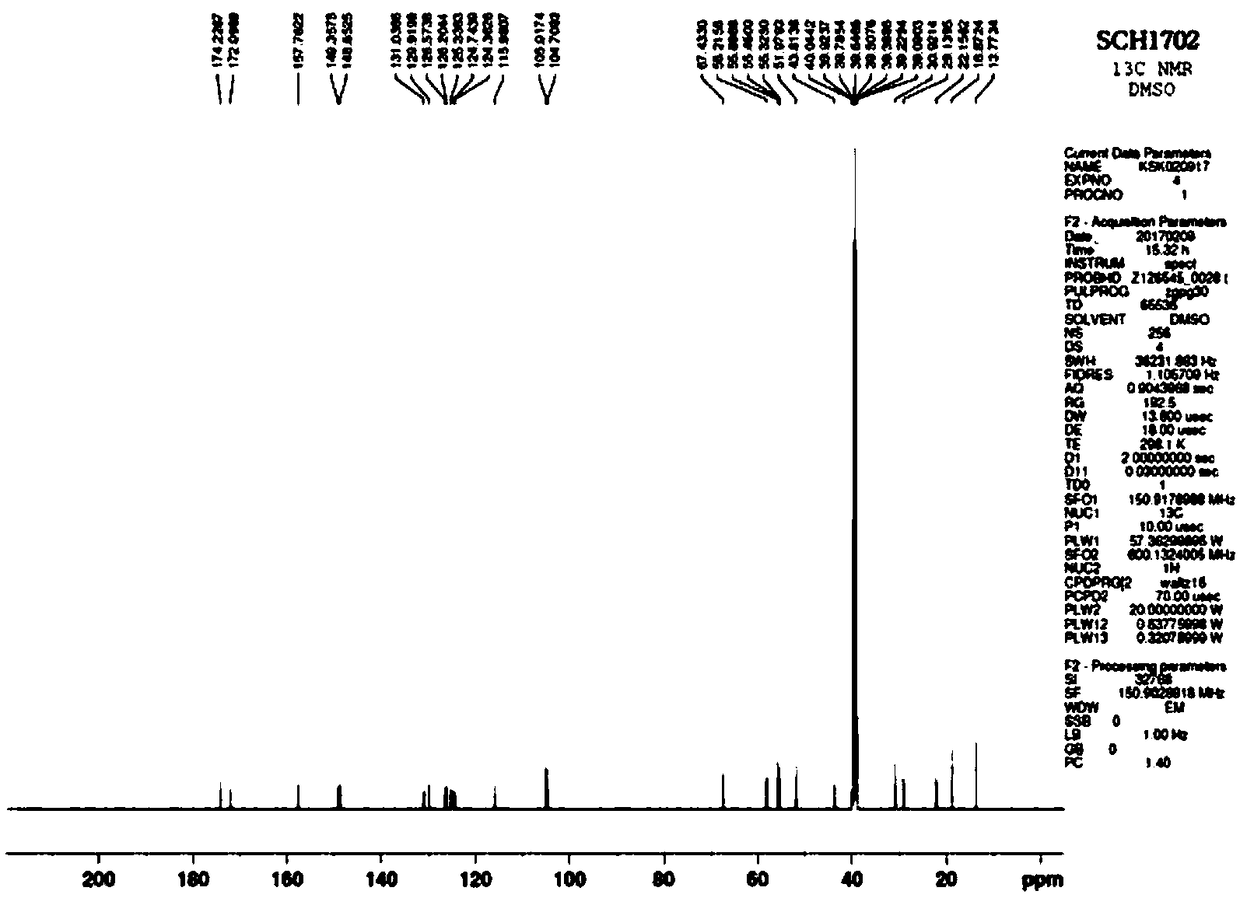
[0080] Preparation Example 1: Synthesis of Compound (SCH1702) of Chemical Formula 2
[0081] The compound of Chemical Formula 2 (SCH1702) was synthesized by the method shown in Reaction Formula 1 below.
[0082] Reaction 1:
[0083]
[0084] In the above reaction formula 1, 3,4-dimethoxyphenylacetic acid (Homoveratricacid) represented by chemical formula A and 4-butoxybenzaldehyde (4-butoxybenzaldehyde) represented by chemical formula B are made with triethylamine ( triethylamine, Et 3 N), acetic anhydride (Acetic anhydride, Ac 2 O) together in N 2 The reaction was carried out under the conditions of 10 hours, filtered with a filter after cooling, and then purified with EtOAc to obtain a yellow compound (Process 1-1).
[0085] Add thionyl chloride (thionyl chloride, SOCl 2 ), made to react for 40 minutes, concentrated in vacuo, removed the solvent, added pyridine (pyridine) and methanol again, and reacted again in the ice tank for 40 minutes to obtain a compound repres...
Embodiment 1
[0097] Example 1: Evaluation of anti-tuberculosis function
[0098] The anti-tuberculosis function was double confirmed by REMA (Resazurin microtiter assay: resazurin microtiter assay) and MGIT method.
[0099] 1) Preparation of Resazurin solution
[0100] Put 4mg of resazurin sodium salt (resazurin sodium salt, sigma-aldrich) into 40ml of sterilized water, after preparing 0.01% resazurin solution, filter with 0.45 μm filter, thus prepared resazurin ( Resazurin) solution, use after refrigerated storage.
[0101] 2) Preparation of tuberculosis and drug-resistant tuberculosis
[0102] H37Ra (Mycobacterium tuberculosis H37Ra: Mycobacterium tuberculosis H37Ra), H37Rv (Mycobacterium tuberculosis H37Rv: Mycobacterium tuberculosis H37Rv), extensively drug-resistant tuberculosis (XDR-TB) bacteria, isoniazid-resistant tuberculosis ( Isoniazid-resistant tuberculosis (INHr-TB) bacteria, Rifampin-resistant tuberculosis (RIFr-TB) bacteria, pyrazinamide-resistant tuberculosis (PZAr-T...
Embodiment 2
[0157] Example 2: Cytotoxicity Evaluation
[0158] Cytotoxicity was measured in THP-1, Raw264.7, L929 and HEK-293 cells associated with tuberculosis infection and proliferation. Take 10x 10 4 Cells / well (cells / well), SCH1702 (S type) were treated in DMEM medium, and cultured for 24 hours and 48 hours. As a new medium, 500 μl of medium and 5 μl of MTT solution were added to a 24-well plate, and further reacted for four hours. After the culture medium was removed, formazan was dissolved in 200 μl of DMSO, and the absorbance was measured at a wavelength of 540 nm using a microplate reader (microplate reader), expressed as 50% toxic concentration (CC 50 , 50% Cytotoxic concentration) marked cytotoxicity, and in Figure 4 The results are shown in . The measured values are all the average values after repeating the experiment three times, and the results are shown. refer to the Figure 4 , it can be confirmed that the compound of SCH1702 (S type) has almost no cytotoxicity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com