Method for preparing palladium pyridine imine (II) catalyst and preparing oil-phase oligomer by catalyzing ethylene
A technology of pyridinium palladium and catalysis of ethylene, which is applied in the field of olefin polymerization to achieve high activity, promote transfer rate, and increase instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of ligand L1:
[0034]
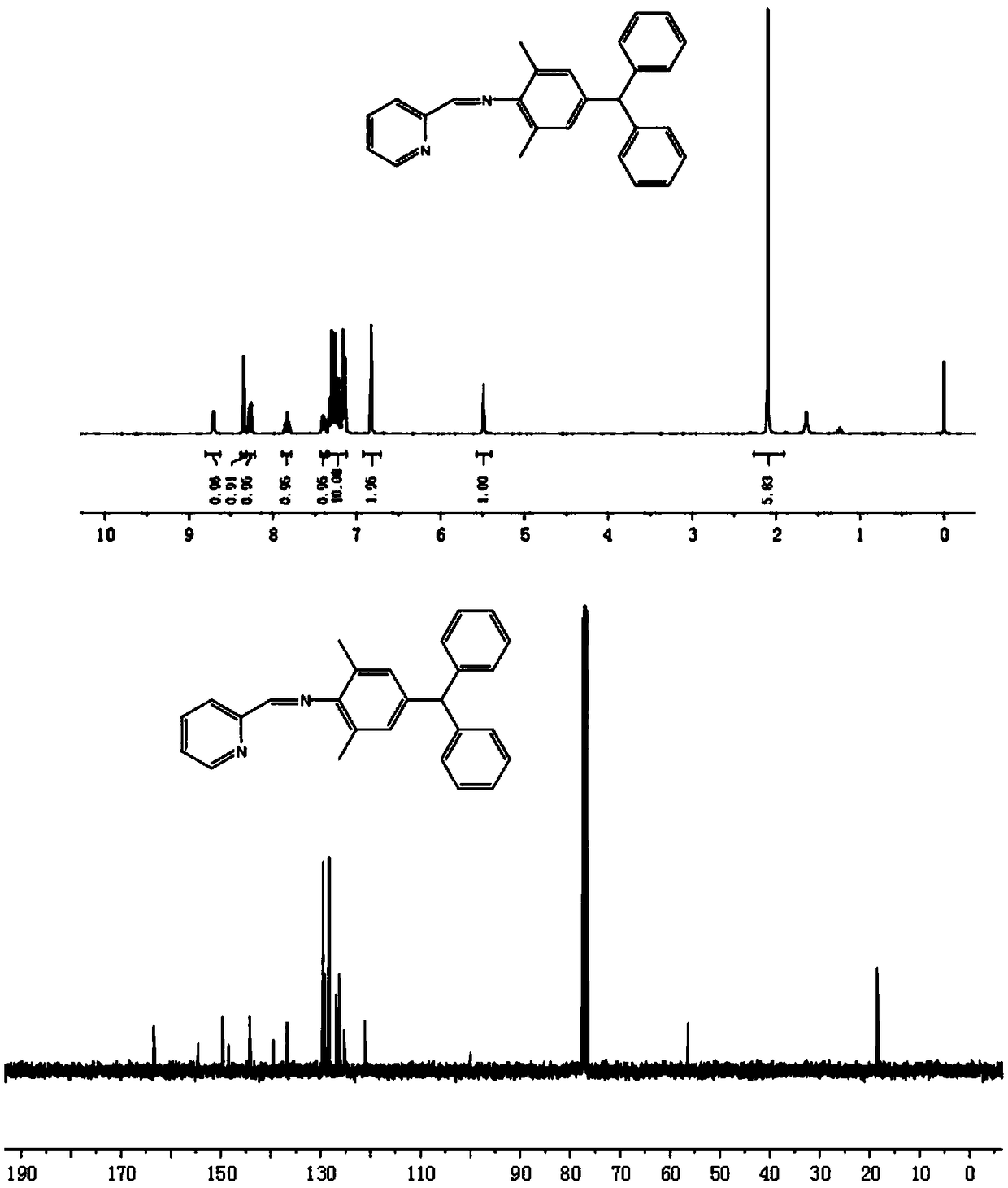
[0035] Dissolve 4-benzyl-2,6-dimethylaniline (0.57g, 2.0mmol) and pyridine-2-carbaldehyde (0.22g, 2.1mmol) in 20mL ethanol, add formic acid (0.2ml ), after refluxing reaction at 50℃ for 24 hours, remove the solvent to obtain the crude product, and then use C 2 H 5 OH / CH 2 Cl 2 (v / v=15:2) The mixed solvent was recrystallized to precipitate a solid precipitate, which was filtered and dried to obtain 0.65 g of the ligand. The yield was 86%.
[0036] 1 HNMR(400MHz, CDCl 3 ,ppm):δ8.71(d,1H,J=6.6Hz,Pr-H),8.35(s,1H,N=CHC 5 H 5 N), 8.27(d,1H,J=7.8Hz,Pr-H),7.86-7.81(m,1H,Pr-H),7.42-7.37(m,1H,Pr-H),7.31-7.14(m ,10H,Ph-H),6.83(s,2H,Ar-H),5.49(s,1H,-CH(Ph) 2 ),2.10(s,6H,-CH 3 ). 13 CNMR(100MHz, CDCl 3 ,ppm):δ163.4(N=CHC 5 H 5 N),154.5,149.6,148.5,144.2,139.5,136.7,129.5,129.2,128.3,126.9,126.2,125.3,121.2,56.4(CH(Ph) 2 ),18.5(-CH 3 ).Anal.Calcd.for C 27 H 24 N 2 (376.19): C, 86.13; H, 6.43; N, 7.44. Found: C, 86.15; H, 6.39; N, 7.42.
[0037] The r...
Embodiment 2
[0047] (1) Synthesis of ligand L2:
[0048]
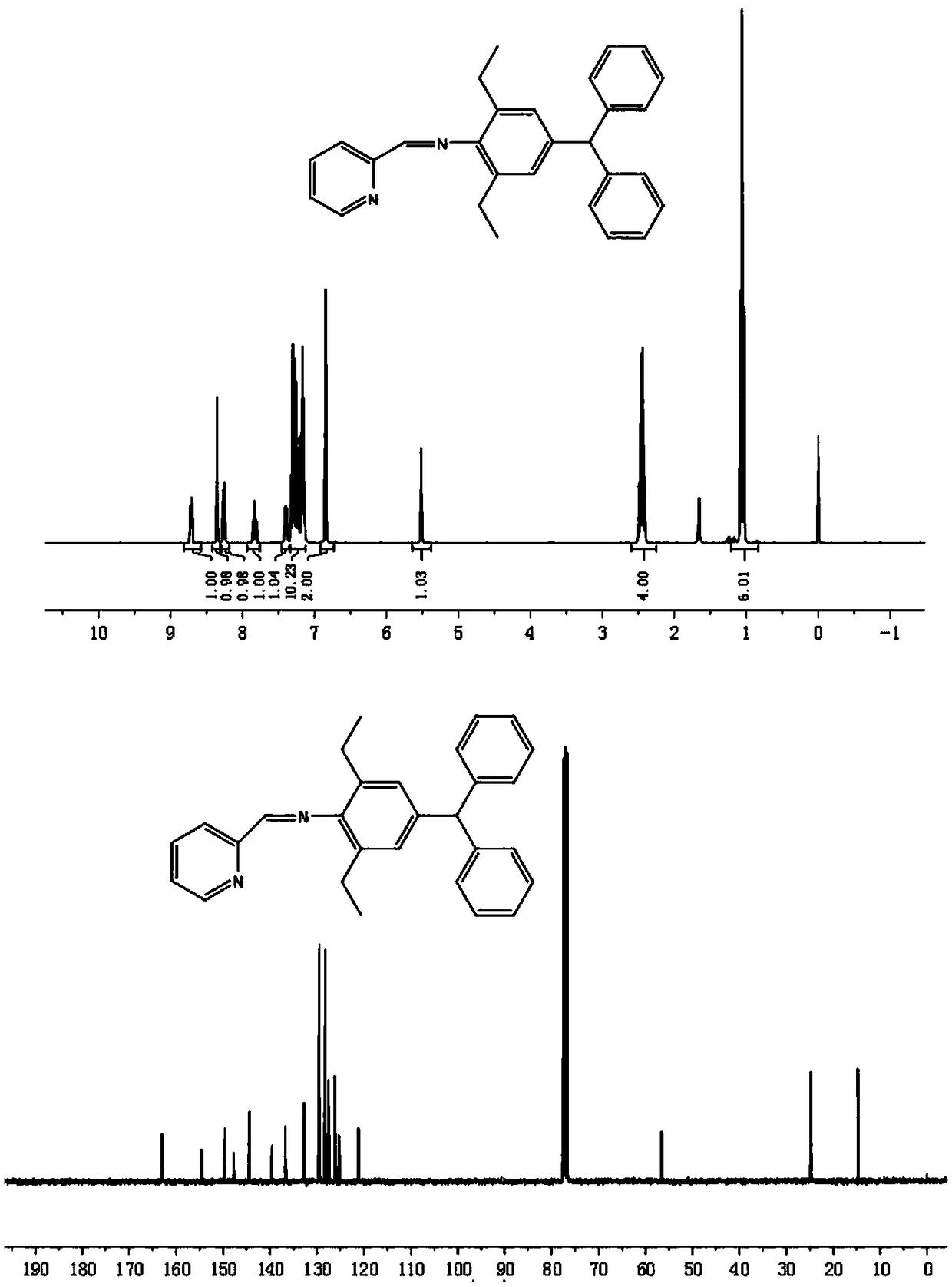
[0049] 4-Diphenylmethyl-2,6-diethylaniline (0.32g, 2.0mmol) and pyridine-2-carboxaldehyde (0.22g, 2.1mmol) were dissolved in 20mL ethanol, and formic acid (0.2ml ), after refluxing at 50℃ for 16 hours, remove the solvent to obtain the crude product, and then use C 2 H 5 OH / CH 2 Cl 2 (v / v=10:2) The mixed solvent was recrystallized to precipitate a solid precipitate, which was filtered and dried to obtain 0.66 g of the ligand. The yield was 82%.
[0050] 1 H NMR(400MHz, CDCl 3 ,ppm):δ8.71(d,1H,J=4.8Hz,Pr-H),8.35(s,1H,N=CHC 5 H 5 N), 8.26(d,1H,J=7.8Hz,Pr-H),7.85-7.81(m,1H,Pr-H),7.42-7.37(m,1H,Pr-H),7.33-7.15(m ,10H,Ph-H),6.85(s,2H,Ar-H),5.52(s,1H,-CH(Ph) 2 ),2.49-2.41(m,4H,-CH 2 CH 3 ),1.06(t,6H,J=7.5Hz,-CH 3 ). 13 C NMR(100MHz, CDCl 3 ,ppm):δ163.0(N=CHC 5 H 5 N), 154.5, 149.6, 147.7, 144.4, 139.6, 136.8, 132.7, 129.5, 128.3, 127.5, 126.2, 125.3, 121.2, 56.6 (CH(Ph) 2 ),24.8(-CH 2 CH 3 ),18.5(-CH 3 ).Anal.Calcd.for C 29 H 28 N 2 (404.23):...
Embodiment 3
[0061] (1) Synthesis of ligand L3:
[0062]
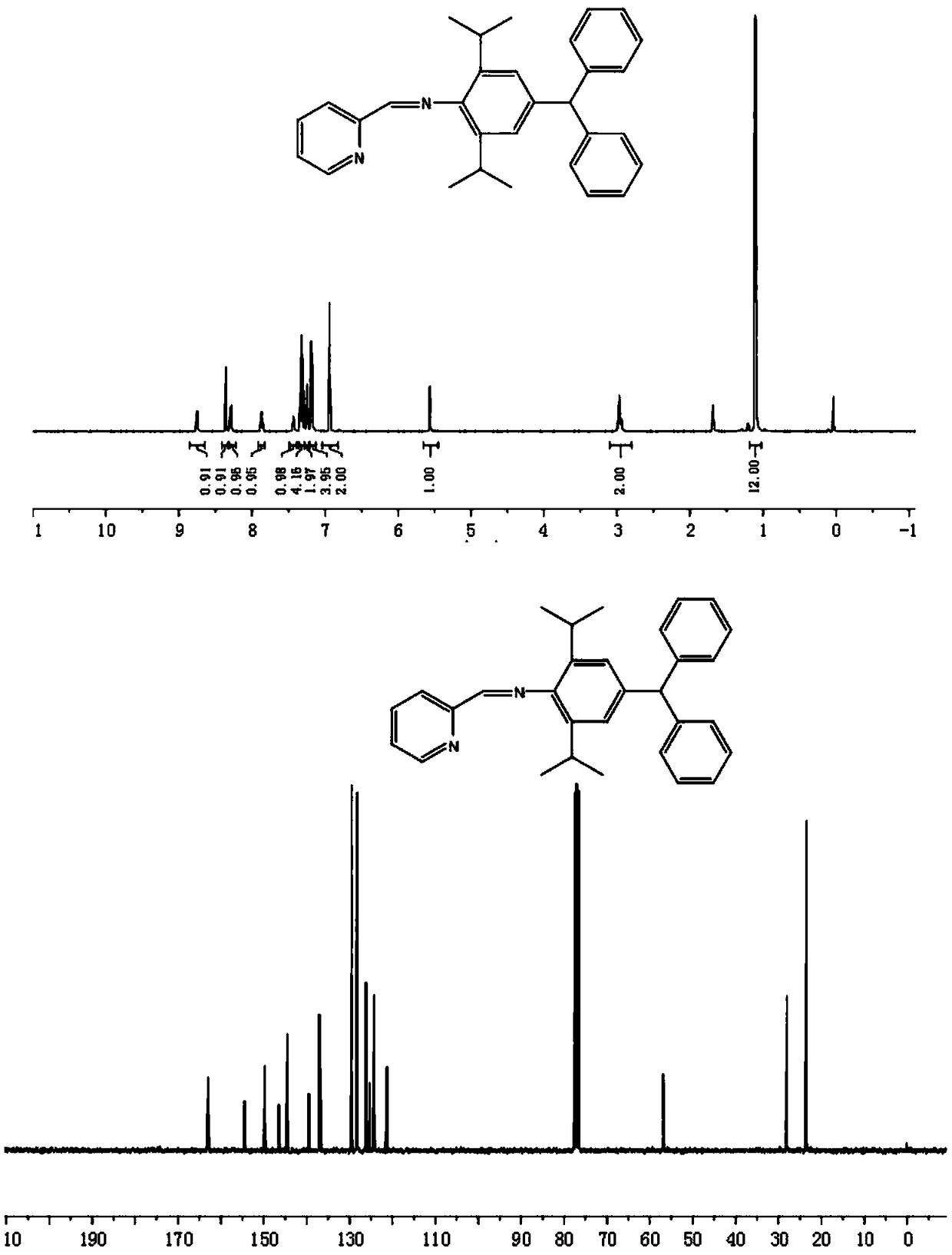
[0063] 4-Diphenylmethyl-2,6-diisopropylaniline (0.69g, 2.0mmol) and pyridine-2-carbaldehyde (0.22g, 2.1mmol) were dissolved in 20mL ethanol, and formic acid (0.2 ml), after refluxing at 50℃ for 16 hours, remove the solvent to obtain the crude product, and then use C 2 H 5 OH / CH 2 Cl 2 (v / v=10:2) The mixed solvent was recrystallized to precipitate a solid precipitate, which was filtered and dried to obtain 0.69 g of ligand. The yield was 80%.
[0064] 1 H NMR(400MHz, CDCl 3 ,ppm):δ8.75(d,1H,J=3.0Hz,Pr-H),8.36(s,1H,N=CHC 5 H 5 N), 8.29(d,1H,J=4.8Hz,Pr-H),7.88-7.85(m,1H,Pr-H),7.44-7.42(m,1H,Pr-H),7.34-7.29(m ,4H,Ph-H),7.26-7.23(m,2H,Ph-H),7.19(d,4H,J=4.2Hz,Ph-H),6.94(s,2H,Ar-H),5.56( s,1H,-CH(Ph) 2 ),2.96(s,2H,-CH(CH 3 ) 2 ), 1.11(d,12H,J=3.9Hz,-CH 3 ). 13 CNMR(100MHz, CDCl 3 ,ppm):δ163.0(N=CHC 5 H 5 N), 154.5, 149.7, 146.5, 144.5, 139.5, 137.1, 136.8, 129.5, 128.2, 126.1, 125.3, 124.3, 121.3, 56.9 (CH(Ph) 2 ),28.0(-CH 3 ),24.5(-CH(CH 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com