Pioglitazone hydrochloride orally disintegrating tablet and preparation method thereof
A technology of pioglitazone hydrochloride and orally disintegrating tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of low dissolution rate, incomplete dissolution, and low bioavailability, etc. problems, to achieve the effects of accelerated dissolution, short disintegration time, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
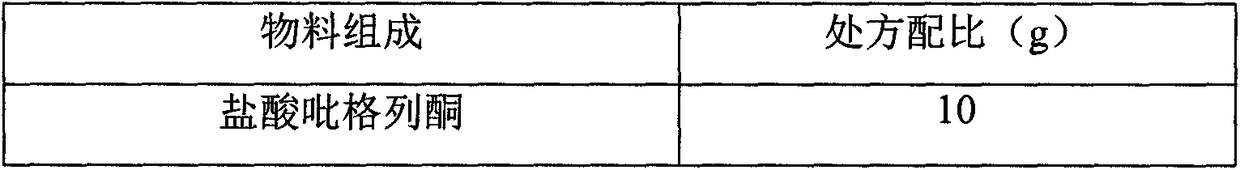
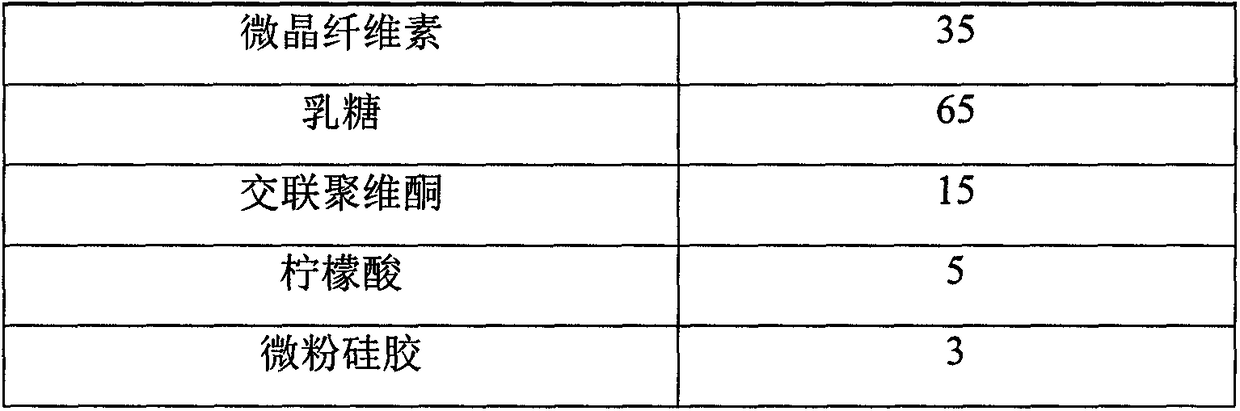
[0012] prescription:
[0013] Material composition
Prescription ratio (g)
Pioglitazone Hydrochloride
10
25
55
Crospovidone
5
1
Micropowder silica gel
0.5
[0014] Preparation method: pioglitazone hydrochloride is micronized by supercritical fluid recrystallization, using supercritical fluid crystallization pharmaceutical equipment (SCF PD Lab type), the steps are as follows: 1) use a pump to input CO2 through the pipeline into the heater, and heat it to become super Critical CO enters in the crystallization kettle, and the temperature in the crystallization kettle is 45 ℃, and the pressure is 11MPa, 2) the pioglitazone hydrochloride solution is input in the above-mentioned crystallization kettle through pipelines with a pump, the concentration of the pioglitazone hydrochloride solution is 2%, and the solvent is methanol: dichloro Methane (volume ratio 1:...
Embodiment 2
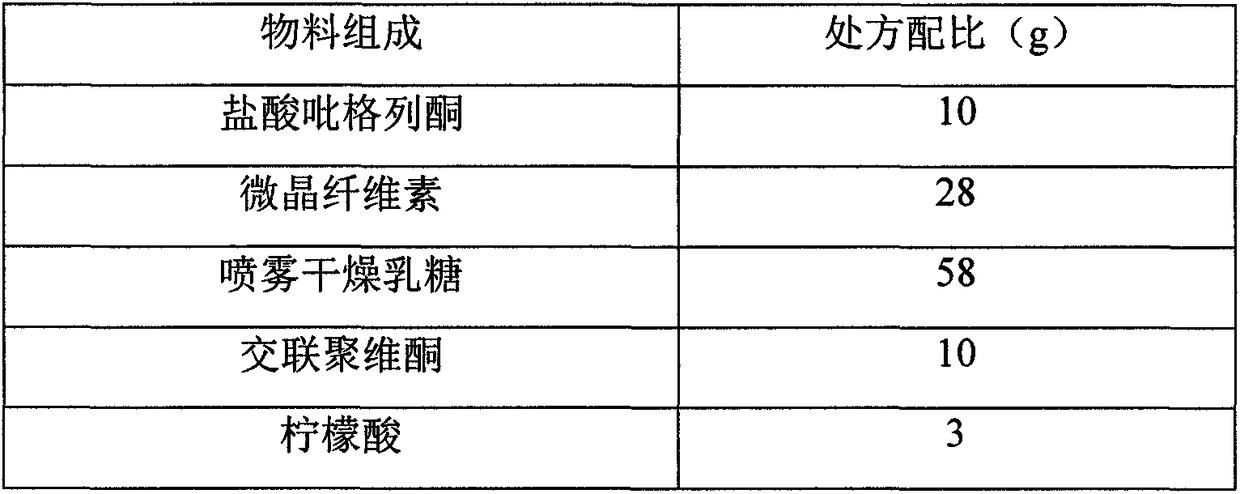
[0017] prescription:
[0018]
[0019]
[0020] Preparation method: pioglitazone hydrochloride is micronized by supercritical fluid recrystallization, using supercritical fluid crystallization pharmaceutical equipment (SCF PD Lab type), the steps are as follows: 1) use a pump to input CO2 through the pipeline into the heater, and heat it to become super Critical CO2 enters in the crystallization kettle, and the temperature in the crystallization kettle is 55 ℃, and the pressure is 14MPa, 2) the pioglitazone hydrochloride solution is input in the above-mentioned crystallization kettle through pipelines with a pump, the concentration of the pioglitazone hydrochloride solution is 4%, and the solvent is methanol: dichloro Methane (volume ratio 2: 1) mixed solvent; 2) in the crystallization kettle, supercritical CO2 and pioglitazone hydrochloride solution are mixed in the nozzle and sprayed out by the nozzle, and the crystallization of pioglitazone hydrochloride is separated o...
Embodiment 3
[0023] prescription:
[0024] Material composition
Prescription ratio (g)
Pioglitazone Hydrochloride
10
35
55
Crospovidone
13
5
Micropowder silica gel
2
[0025] Preparation method: pioglitazone hydrochloride is micronized by supercritical fluid recrystallization, using supercritical fluid crystallization pharmaceutical equipment (SCF PD Lab type), the steps are as follows: 1) use a pump to input CO2 through the pipeline into the heater, and heat it to become super Critical CO enters in the crystallization kettle, and the temperature in the crystallization kettle is 50 DEG C, and the pressure is 18MPa, 2) the pioglitazone hydrochloride solution is input in the above-mentioned crystallization kettle through pipelines with a pump, the concentration of the pioglitazone hydrochloride solution is 4%, and the solvent is methanol: dichloro Methane (volume ratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com