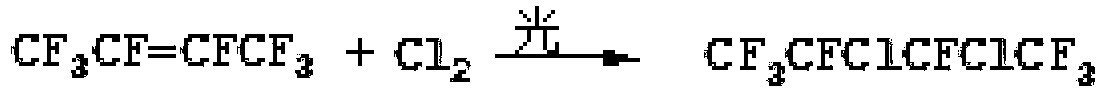Method for preparing dichlorooctafluorobutane by means of liquid-phase photochlorination
A technology of dichlorooctafluorobutane and chlorination method, which is applied in the fields of chemical instruments and methods, preparation of halogenated hydrocarbons, preparation of halogen addition, etc. It can solve the problem of relatively harsh reaction conditions, easy polymerization and purification of perfluorobutene Difficult separation and other problems, to achieve the effect of simple and efficient preparation, avoid high temperature self-polymerization, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Put 2000mol of perfluoro-2-butene into a 500L enamel reactor equipped with a light source irradiation port, under the irradiation of a light source with a power of 1kw and a wavelength of 400nm, continuously feed 2000mol of chlorine gas for reaction, and the reaction temperature is controlled at -10°C , the reaction pressure was controlled at 20kPa, and the material was discharged after the reaction was completed, washed with alkali, dried, and purified by rectification to obtain 1992mol of product 2,3-dichlorooctafluorobutane, with a yield of 99.6%.
Embodiment 2
[0031] Put 2000mol of perfluoro-2-butene into a 500L enamel reaction tower equipped with a light source irradiation port. Under the irradiation of a light source with a power of 2kw and a wavelength of 315nm, 3000mol of chlorine gas is continuously introduced to react. The reaction temperature is controlled at 0°C. The pressure was controlled at 60kPa, and the material was discharged after the reaction was completed, washed with alkali, dried, and purified by rectification to obtain 1996mol of the product 2,3-dichlorooctafluorobutane, with a yield of 99.8%.
Embodiment 3
[0033] Put 2000mol of perfluoro-2-butene into a 500L enamel reaction tower equipped with a light source irradiation port. Under the irradiation of a light source with a power of 3kw and a wavelength of 250nm, 4000mol of chlorine gas is continuously introduced to react. The reaction temperature is controlled at 10°C. The pressure was controlled at 100kPa, and the material was discharged after the reaction was completed, washed with alkali, dried, and purified by rectification to obtain 1974mol of the product 2,3-dichlorooctafluorobutane, with a yield of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com