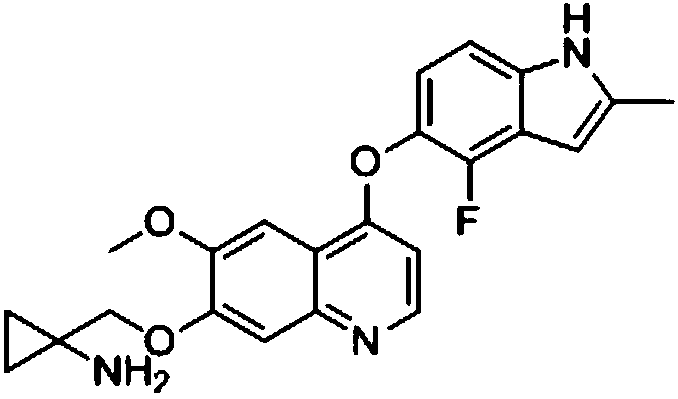
Method for synthesizing anlotinib and hydrochloride thereof
A synthesis method and technology of cyclopropylamine hydrochloride, applied in the field of pharmaceutical synthesis, can solve problems such as complicated operation, many steps, poor product solubility, etc., and achieve the effects of simple reaction operation, improved reaction efficiency, and simplified technological process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 1-((4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropylamine Synthesis of hydrochloride:
[0034]
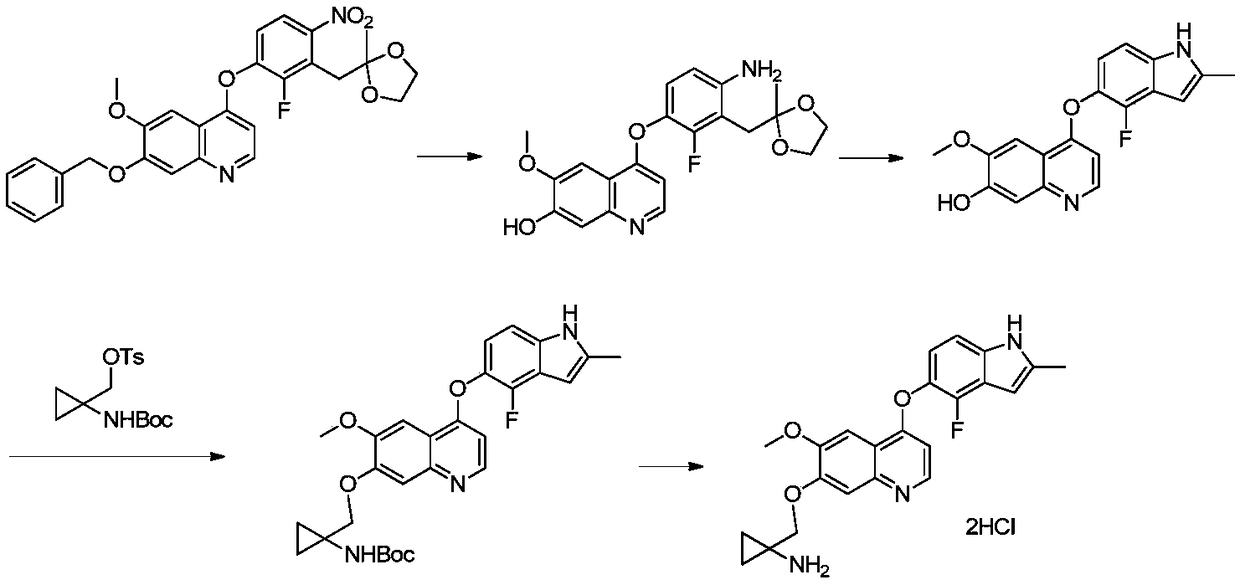
[0035] 1. Preparation of 4-(4-amino-2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl)phenoxy)-6-methoxyquinoline Lino-7-ol:
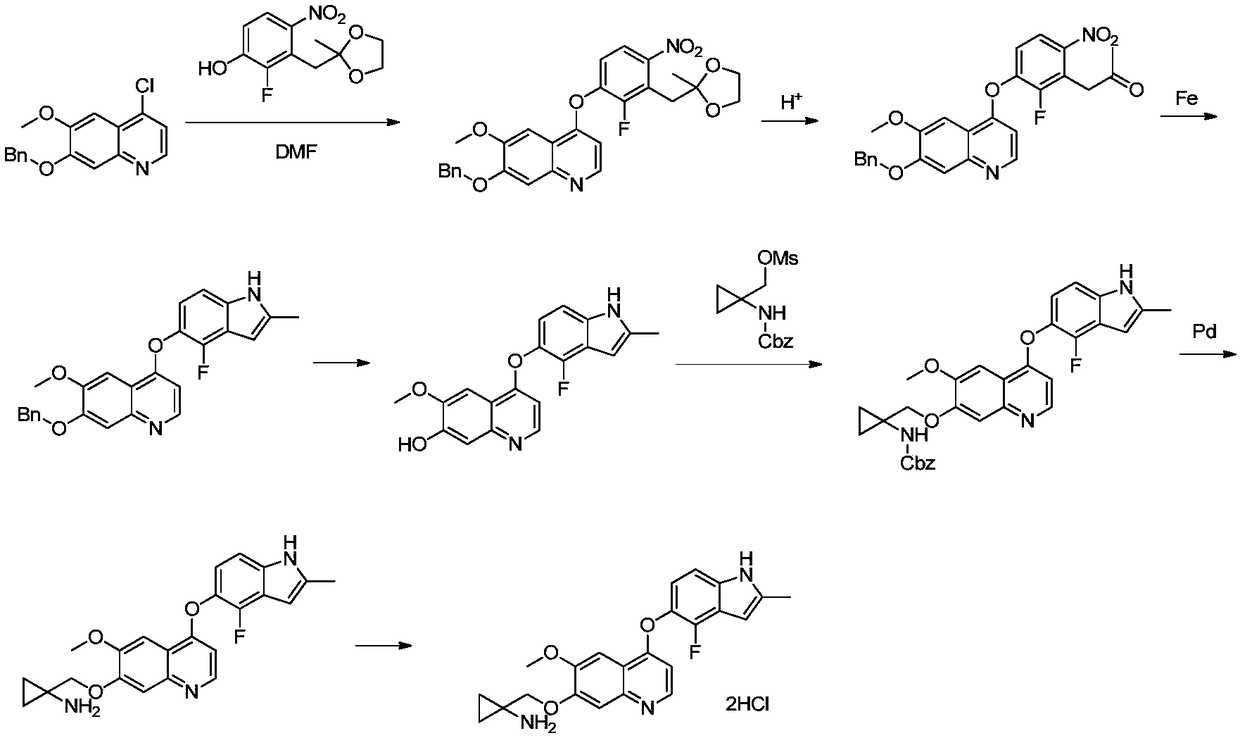
[0036] 7-(benzyloxy)-4-(2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl)-4-nitrophenoxy) -6-Methoxyquinoline (52g, 0.1mol) was placed in a 500mL there-necked flask, 280mL of N-methylpyrrolidone was added, and then wet palladium carbon (5g, ~10wt%) was added, under 0.1 ~ 0.2MPa hydrogen pressure The reaction was carried out at 50-55°C until the reaction was completed as detected by HPLC. The catalyst was removed by filtration, and the resulting toluene was distilled off from the mother liquor under reduced pressure to obtain 4-(4-amino-2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl) The solution of phenoxy)-6-methoxyquinolin-7-ol in N-methylpyrrolidone was used directly in the next reaction.
[0037] 1...
Embodiment 2
[0047] Example 2 1-((4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropylamine Synthesis:
[0048]
[0049] 1. Preparation of 4-(4-amino-2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl)phenoxy)-6-methoxyquinoline Lino-7-ol:
[0050] 7-(benzyloxy)-4-(2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl)-4-nitrophenoxy) -6-Methoxyquinoline (52g, 0.1mol) was placed in a 500mL three-necked flask, 280mL of N,N-dimethylacetamide was added, and then wet palladium carbon (5g, ~10wt%) was added, at 0.1-0.2 Under the hydrogen pressure of MPa, the reaction was carried out at 50-55 °C until the reaction was completed as detected by HPLC. The catalyst was removed by filtration, and the resulting toluene was distilled off from the mother liquor under reduced pressure to obtain 4-(4-amino-2-fluoro-3-((2-methyl-1,3-dioxolan-2-yl)methyl) Phenoxy)-6-methoxyquinolin-7-ol in N,N-dimethylacetamide was used directly in the next reaction.
[0051] 2. Preparation of 4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com