Novel chlorin e6 derivative and preparation method and application thereof
A technology for chlorin and derivatives, which is applied in the field of novel chlorin e6 derivatives and their preparation, can solve the problems of high cost, difficulty in popularization and application, difficulty in separation and preparation, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
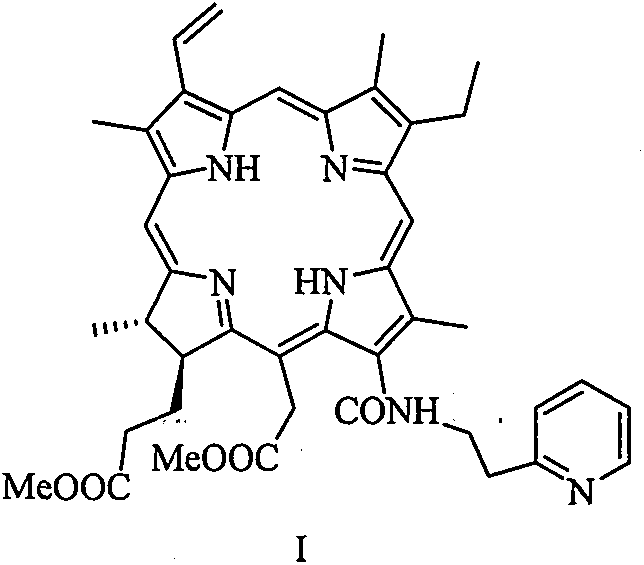
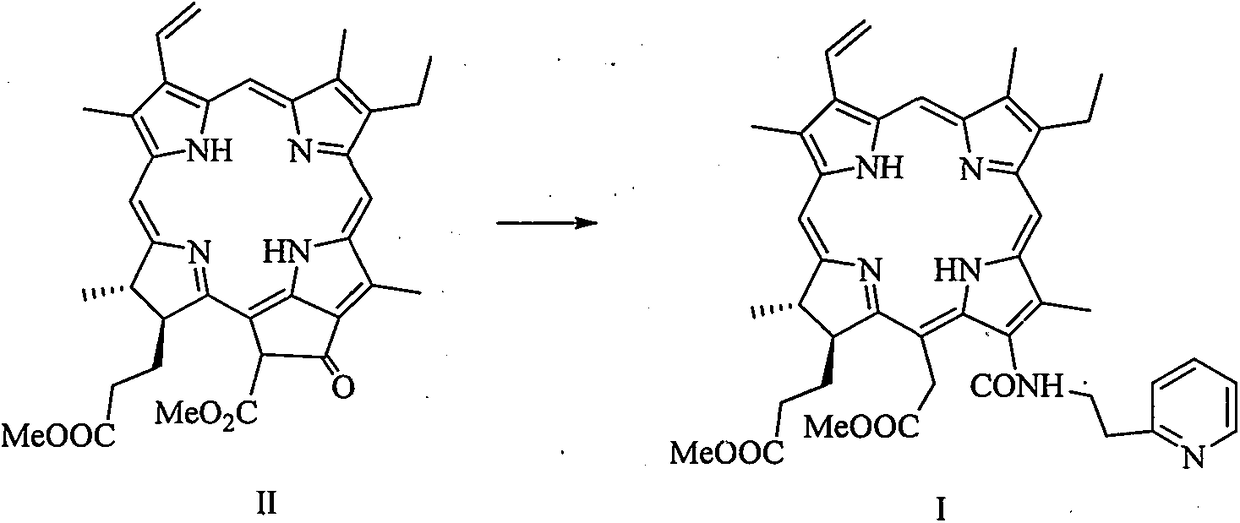
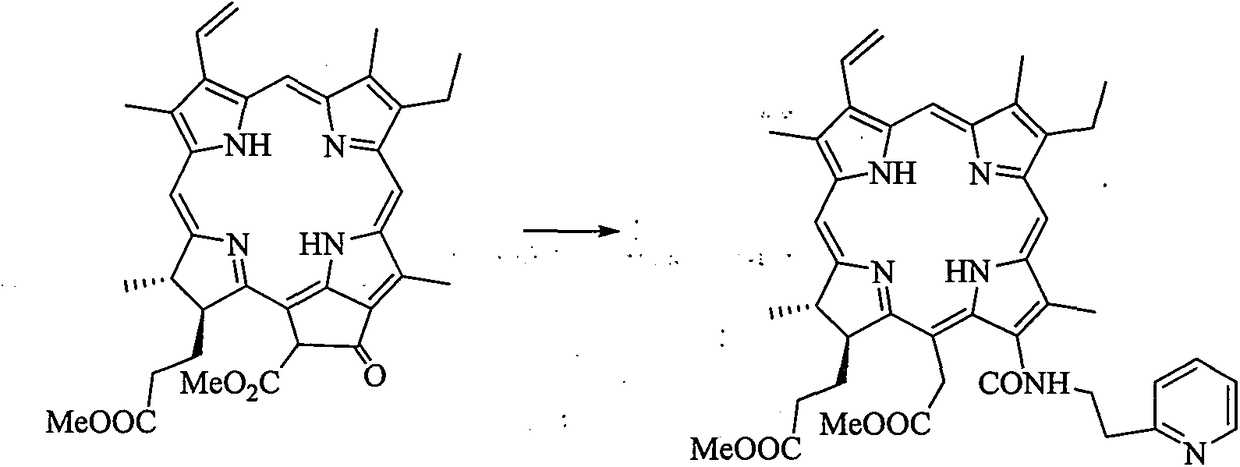
[0020] 1, 13 1 Preparation of -N-[2-(2-pyridine)ethyl]chlorphine e6 amide dimethyl ester
[0021]
[0022] In a 25mL three-necked flask, pheophorbide-a methyl ester (100mg, 0.18mmol), tetrahydrofuran (4mL) and 2-pyridylethylamine (2mL) were stirred at room temperature for 16h, and the reaction was monitored by thin-layer chromatography until the reaction was complete . 40 mL of dichloromethane was added to the reaction solution for extraction. The organic phase was washed with water (20 mL×3), washed with saturated brine (20 mL×3), dried over anhydrous sodium sulfate, and filtered. Concentrated under reduced pressure to remove the solvent, and separated by silica gel column chromatography (methanol / dichloromethane=1:10) to obtain 13 1 -N-[2-(2-Pyridine)ethyl]chlorin e6 amide dimethyl ester green solid powder (43.8 mg), yield about 38%. 1 H NMR (400MHz, CDCl 3 ): δppm 9.65 (1H, s, H10), 9.62 (1H, s, H5), 8.79 (1H, s, H20), 8.39 (1H, d, J=4.8Hz, H13 6 ), 8.07 (1H, dd, J...
Embodiment 2
[0024] 1. Photodynamic anti-proliferation experiment of photosensitizers on human esophageal cancer cell line Eca-109
[0025] Tested cells: human esophageal cancer cells (Eca-109)
[0026] Drugs tested: 13 1 -N-[2-(2-pyridine)ethyl]chlorin e6 amide dimethyl ester (hereinafter referred to as photosensitizer 1), temoporfin (comparative drug, provided by Shanghai Xianhui Pharmaceutical Technology Co., Ltd.).
[0027] Light source: XD-650AB laser; SD2490 laser power measuring instrument.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com