Oxo-bridged bicyclo-heptylene sulfonamides compound containing different alkyl chain lengths, as well as preparation method and application thereof
A cycloheptene sulfonamide and compound technology, applied in the application field of anti-breast cancer drugs, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] Synthesis of 3-(4-hydroxyphenyl)-4-alkoxyphenylfuran derivatives
[0097] Synthesis of p-methoxybromoacetophenone compound 2
[0098] Weigh p-methoxyacetophenone 1 (3.499g, 23.3mmol), p-toluenesulfonic acid (0.809g, 4.65mmol) and N-bromosuccinimide (5.02g, 27.9mmol) in a 150mL round bottom In the flask, add 30mL of chloroform, react at room temperature for 12h, TLC monitors that the reaction is complete, add water to quench, extract with dichloromethane (3×15ml), dry the organic layer over anhydrous sodium sulfate, desolvate under reduced pressure, and purify through a silica gel column. 3.2 g (60%) of a yellow solid were obtained.
[0099] Synt...
Embodiment 2
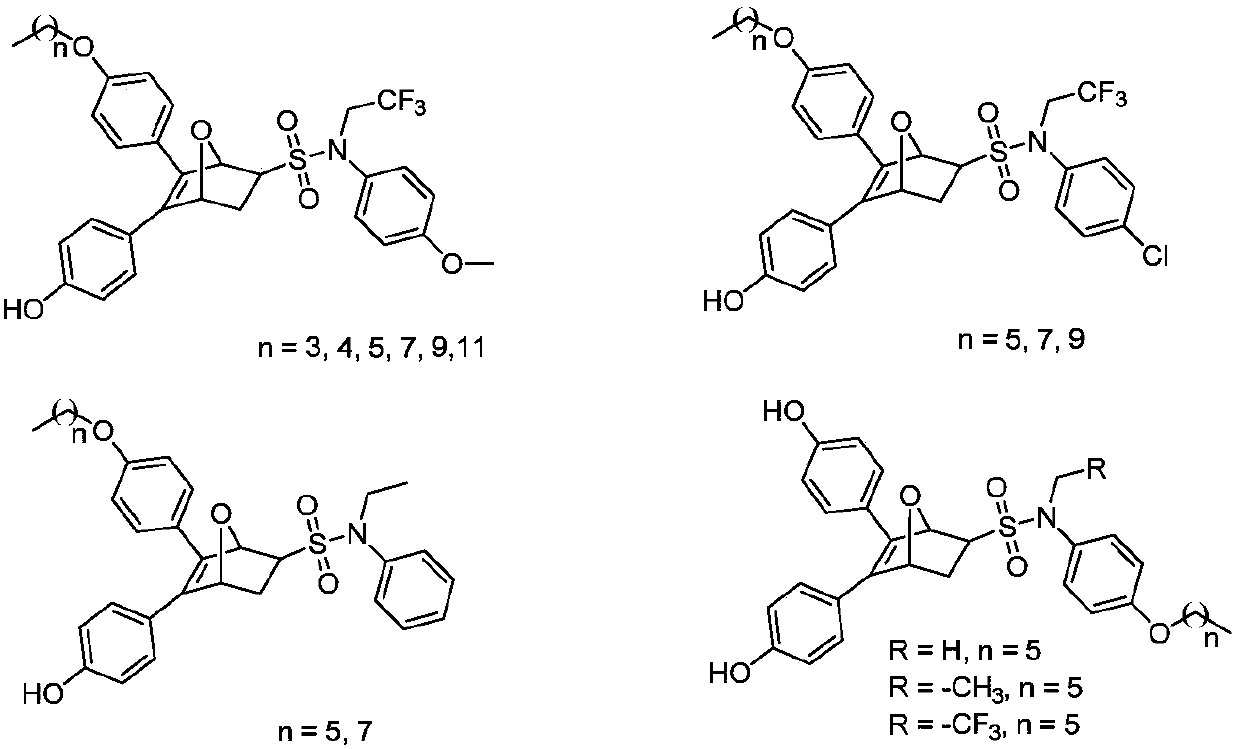
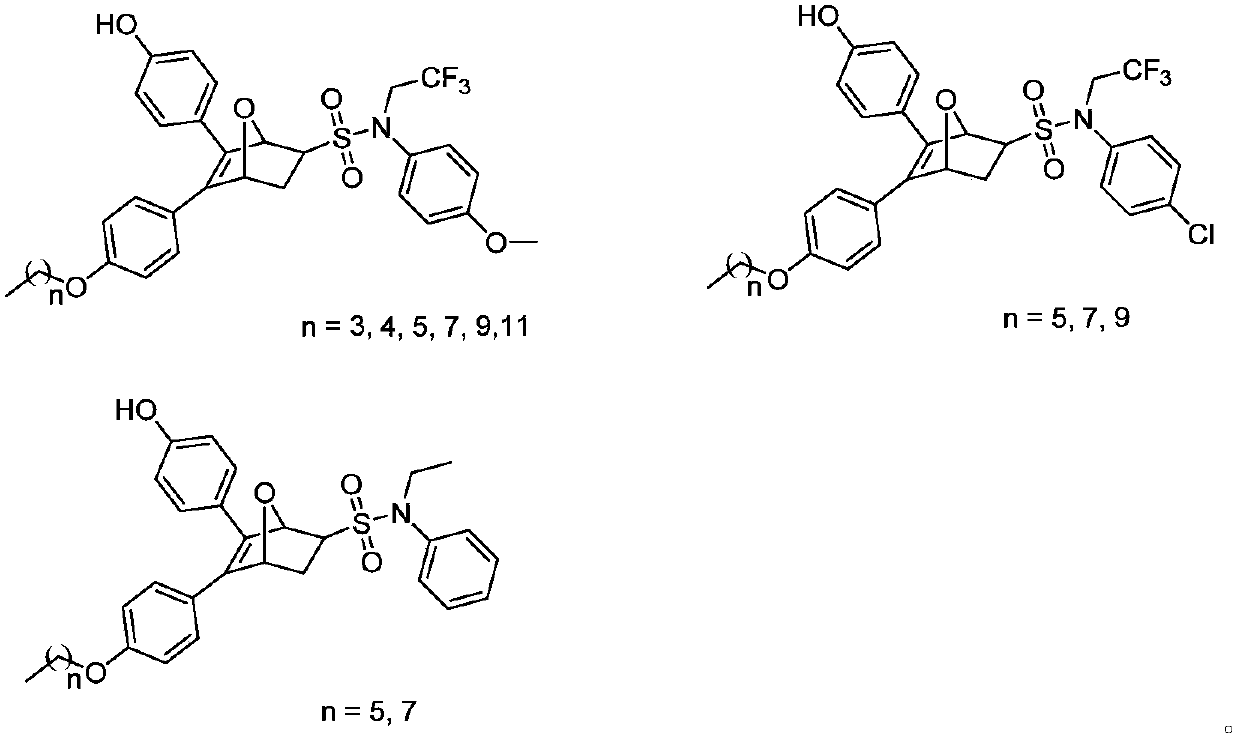
[0110] 5-(4-butoxyphenyl)-6-(4-hydroxyphenyl)-N-(4-methoxyphenyl)-N-(2,2,2-trifluoroethyl)-7 -Oxy-bridge bicyclo[2.2.1]-5-heptene-2-sulfonamide (21a-1),
[0111] 5-(4-hydroxyphenyl)-6-(4-butoxyphenyl)-N-(4-methoxyphenyl)-N-(2,2,2-trifluoroethyl)-7 -Oxy-bridged bicyclo[2.2.1]-5-heptene-2-sulfonamide (21a-2)
[0112]
[0113] Weigh 3-(4-hydroxyphenyl)-4-(4-butoxyphenyl)furan compound 8a (132mg, 0.43mmol) and N-(4-methoxyphenyl)-N-(2, 2,2-Trifluoroethyl)vinylsulfonamide compound 12a (152mg, 0.52mmol) was placed in a 25ml two-necked round-bottomed bottle, and 3ml of anhydrous THF was added to aid dissolution, then the temperature was slowly raised to 90°C, and the reaction was carried out for 8 hours, and the reaction was detected by TLC complete, quenched with water, extracted with ethyl acetate, took the organic layer and dried over anhydrous sodium sulfate. Desolvation under reduced pressure, separation and purification by column chromatography, the eluent ratio is petrol...
Embodiment 3
[0115] 5-(4-pentyloxyphenyl)-6-(4-hydroxyphenyl)-N-(4-methoxyphenyl)-N-(2,2,2-trifluoroethyl)-7 -Oxy-bridged bicyclo[2.2.1]-5-heptene-2-sulfonamide (21b-1),
[0116] 5-(4-Hydroxyphenyl)-6-(4-pentyloxyphenyl)-N-(4-methoxyphenyl)-N-(2,2,2-trifluoroethyl)-7 -Oxy-bridged bicyclo[2.2.1]-5-heptene-2-sulfonamide (21b-2)
[0117]
[0118] Weigh 3-(4-hydroxyphenyl)-4-(4-pentyloxyphenyl)furan compound 8b (145mg, 0.45mmol) and N-(4-methoxyphenyl)-N-(2, 2,2-Trifluoroethyl)vinylsulfonamide compound 12a (159mg, 0.54mmol) was placed in a 25ml two-necked round-bottomed bottle, and 3ml of anhydrous THF was added to aid dissolution, then the temperature was slowly raised to 90°C, and the reaction was carried out for 8 hours, and the reaction was detected by TLC complete, quenched with water, extracted with ethyl acetate, took the organic layer and dried over anhydrous sodium sulfate. Desolvation under reduced pressure, separation and purification by column chromatography, the eluent ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com