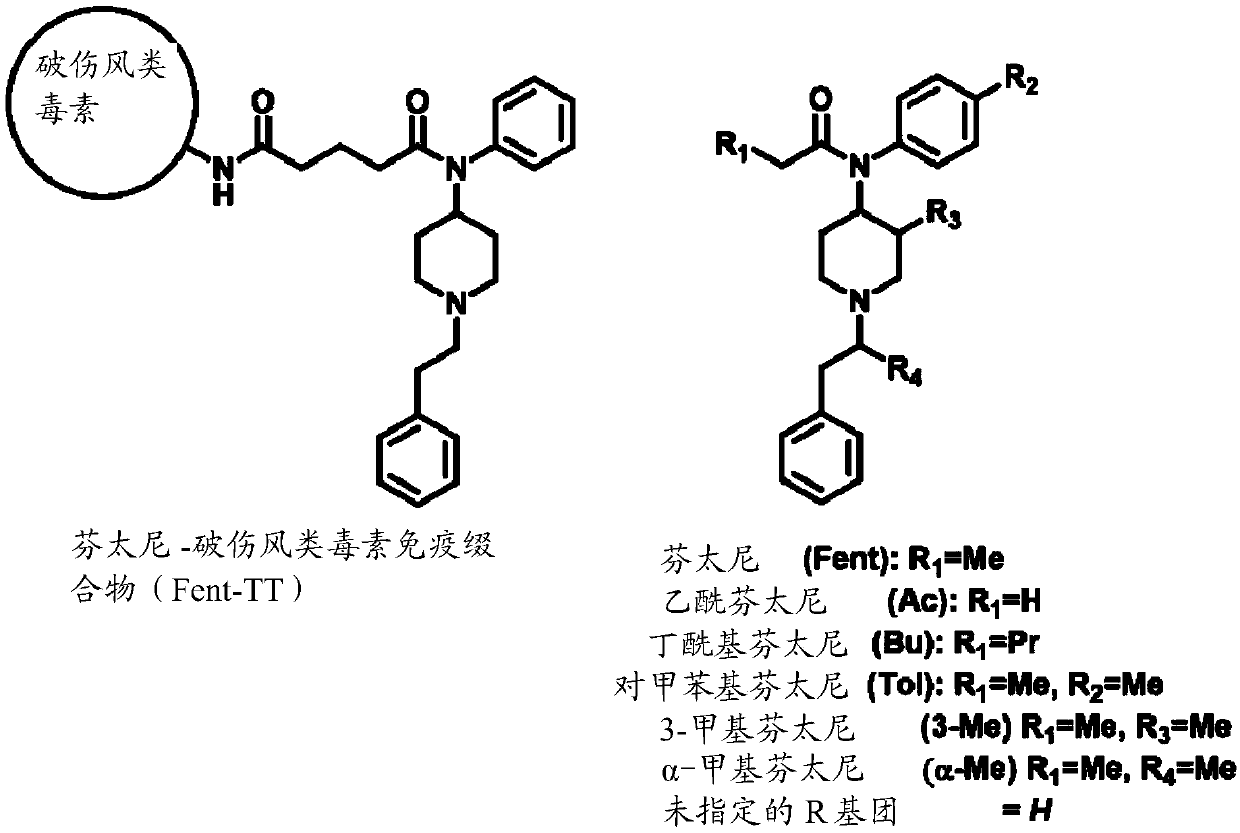
Synthetic opioid vaccine
A vaccine, conjugation technology, in the field of synthetic opioid vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
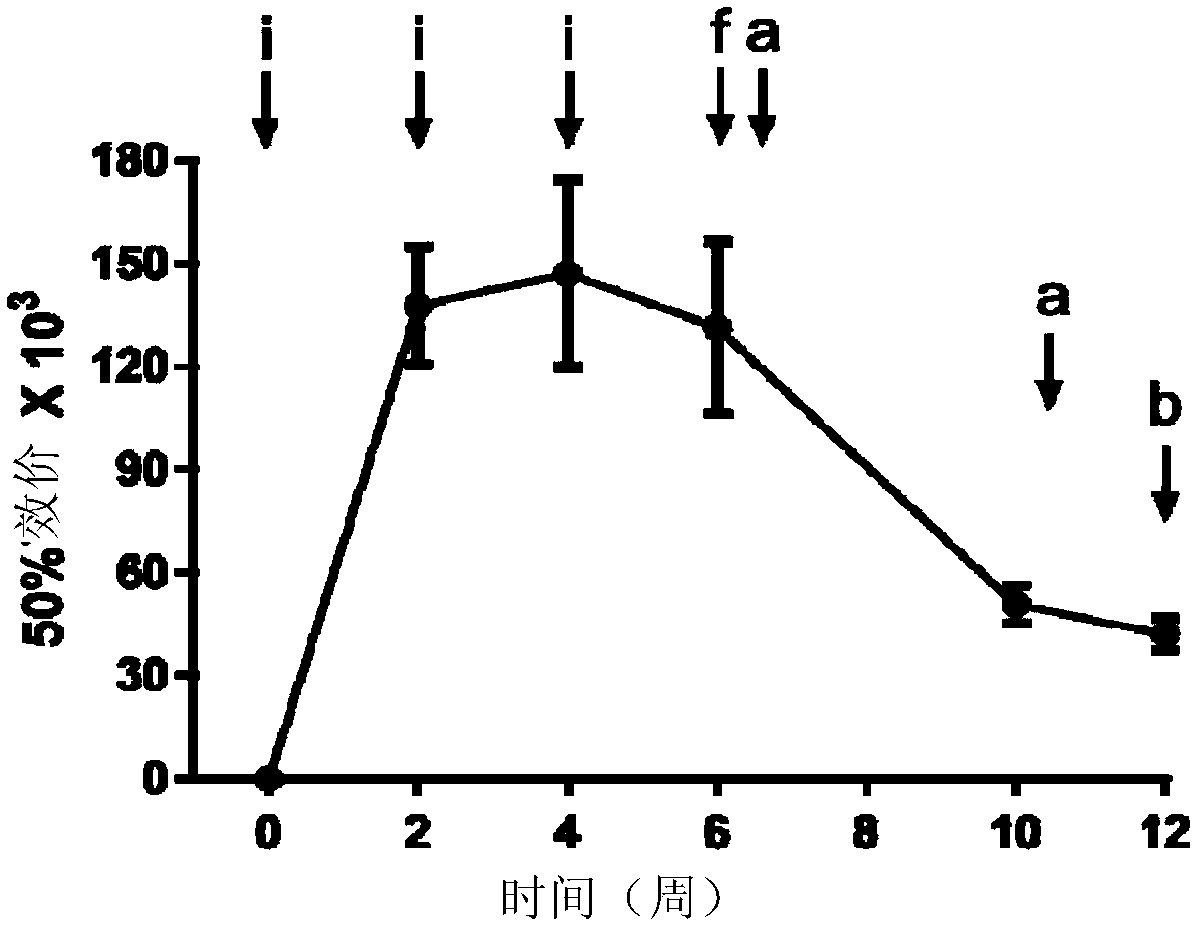
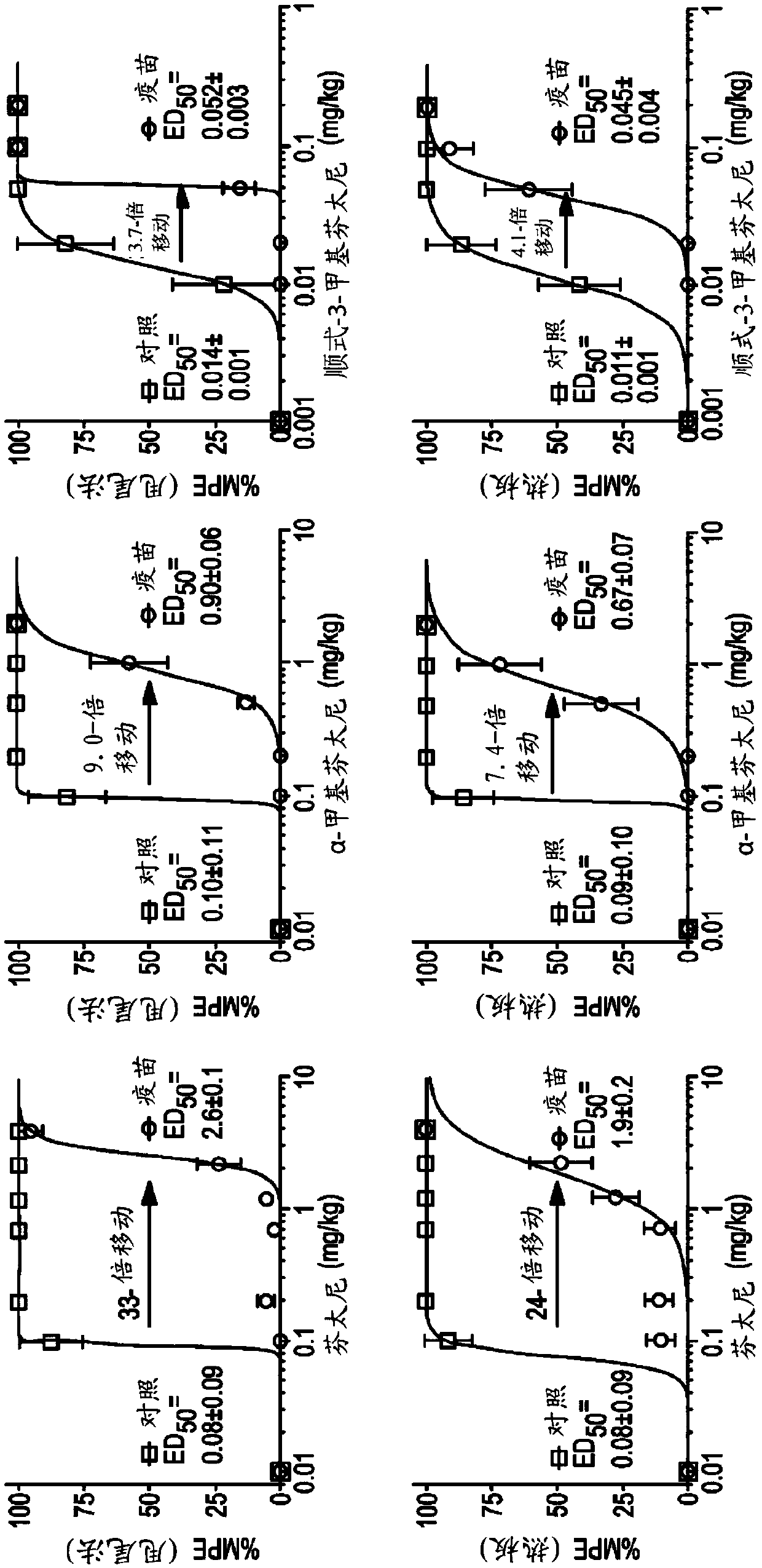
Image
Examples
Embodiment
[0058] Chemical
[0059] Unless otherwise stated, NMR was measured on a Bruker 400 instrument ( 1 H NMR (400MHz), 13 C NMR (100 MHz)) spectrum. 1 H NMR chemical shifts are reported in parts per million (ppm) relative to chloroform (7.26 ppm), and coupling constants are expressed in Hertz (Hz). The following abbreviations are used for spin multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. 13 C NMR chemical shifts are reported in ppm relative to the triplet centerline at 77.0 ppm for chloroform. Electrospray ionization (ESI) masses were acquired on a ThermoFinnigan LTQ Ion Trap. Matrix-assisted laser desorption / ionization (MALDI) mass spectra were acquired on an Applied Biosystems Voyager DE. Assay plates pre-coated in Merck (0.25 mm thick, silica gel 60F 254 ) by analytical thin-layer chromatography (TLC). In pre-coated with silicone 60F 254 Preparative TLC (PTLC) separations were performed on Merck analytical plates (0.50 mm t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com