Preparation method of anhydrous lithium tetraborate
A technology of anhydrous lithium tetraborate and boron compounds, applied in borates, chemical instruments and methods, inorganic chemistry, etc., to achieve the effects of low energy consumption, convenient large-scale industrial implementation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
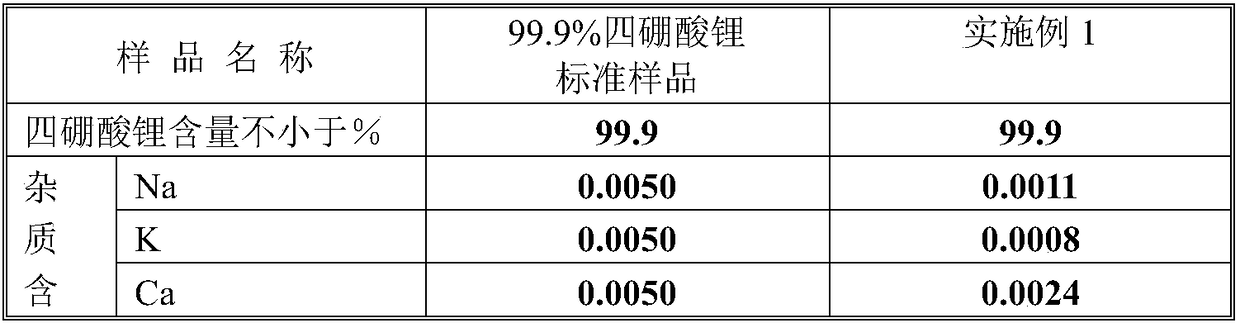
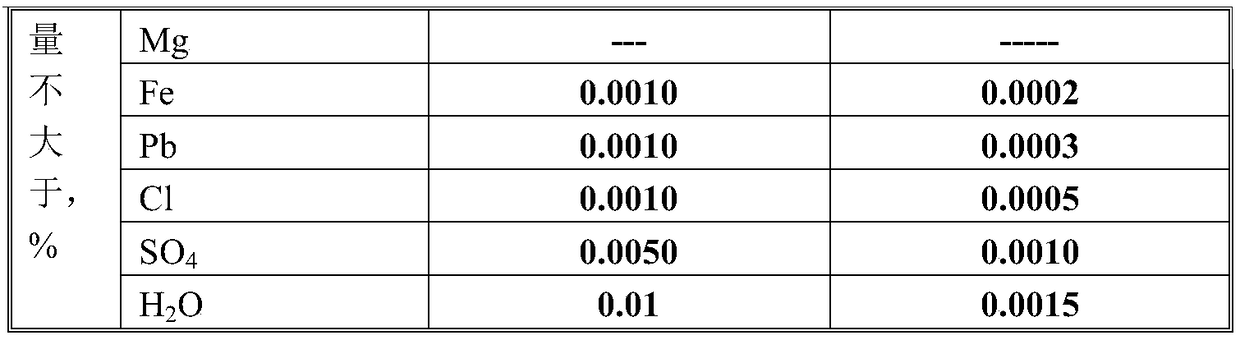
[0025] The fine powder lithium carbonate used in this embodiment has a content of 99.9%; boric acid is analytically pure and has a content of 99.9%.
[0026] (1) Add 7407g of 99.9% lithium carbonate to the 1000L stainless steel double cone mixer;
[0027] (2) Add 25000g of analytical pure boric acid to the stainless steel double cone mixer;
[0028] (3) Close the valves of the double cone, open the double cone speed regulator to start mixing the raw materials, control the rotation speed to 50 revolutions / min, and the mixing time to 120 minutes. After mixing, place the mixture evenly in 10 stainless steel plates. , That is, put 3240g of mixture in each stainless steel pan;
[0029] (4) Put 10 stainless steel pans in a high temperature oven, and control the heating rate of the oven to 10°C / min. When the temperature reaches 200°C, stop the temperature rise and maintain 200°C for 4 hours, then 10°C / min After 3 hours after heating to maintain 500℃, close the oven and cool to room temperat...
Embodiment 2
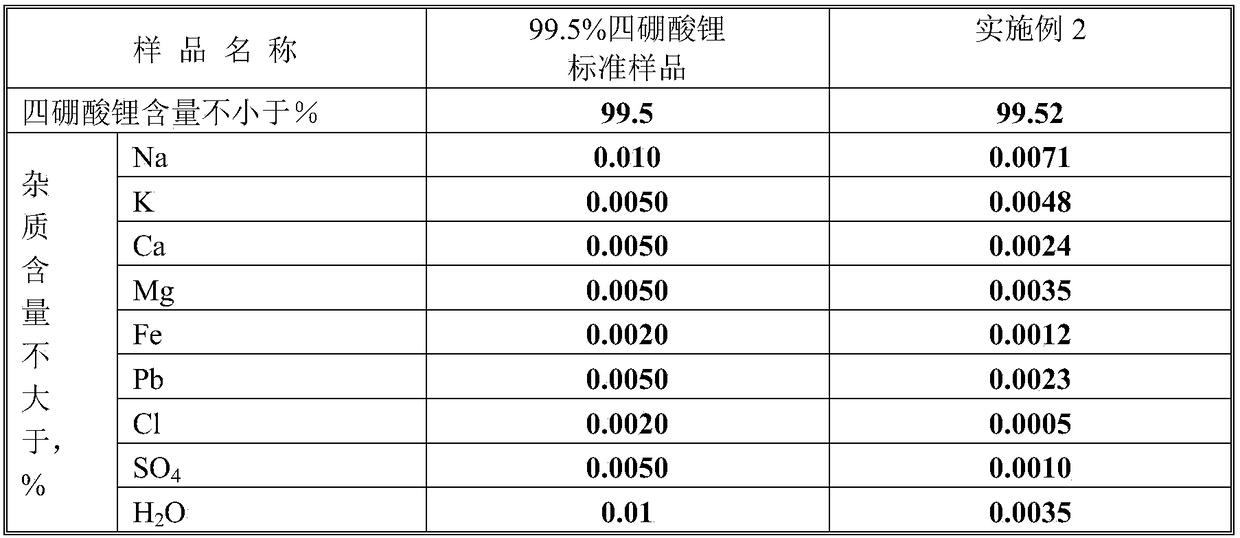
[0036] The micropowder lithium hydroxide monohydrate used in this example is 99.5%; boric acid is analytically pure, with a content of 99.5%.
[0037] (1) Add 42.17kg of 99.5% micropowder monohydrate lithium hydroxide into the 1000L stainless steel double cone mixer;
[0038] (2) Add 130kg of analytical pure boric acid to the stainless steel double cone mixer;
[0039] (3) Close the valves of the double cone, open the double cone governor to start mixing the raw materials, control the rotation speed to 30 revolutions / min, and the mixing time to 150 minutes. After mixing, place the mixture evenly in 20 stainless steel plates. , That is, 8.6085kg of mixed material is placed in each stainless steel pan;
[0040] (4) Put 8 stainless steel pans in a high-temperature oven, and control the heating rate of the oven to 5°C / min. When the temperature reaches 250°C, stop the temperature rise, and maintain 250°C for 3h, and then use 5°C / min. Min heating up to 500℃, stop heating, after maintaining...
Embodiment 3
[0046] The raw materials used in this example are 99.9% finely powdered lithium carbonate and analytically pure boron oxide with a content of 99.5%.
[0047] (1) Add 370.37kg of 99.9% lithium carbonate to the 1000L stainless steel double cone mixer;
[0048] (2) Add 710kg of analytically pure boron oxide to the stainless steel double cone mixer;
[0049] (3) Close the valves of the double cone, open the double cone speed regulator to start mixing the raw materials, control the rotation speed to 20 revolutions / min, and the mixing time to 200 min. After mixing, place the mixture in batches and evenly in the stainless steel plate. , Put 12kg of mixture in each stainless steel pan;
[0050] (4) Put 8 stainless steel pans in a high-temperature oven, and control the heating rate of the oven to be 3℃ / min. When the temperature reaches 240℃, stop the heating, and maintain 240℃ for 3h, then 3℃ / min. Min heating up to 500℃, stop heating, maintain 500℃ for 4h, turn off the oven, and cool to room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com