Asymmetric metal complex black dye and preparation method thereof
A technology of metal complexing and black dyes, applied in the field of dyes, can solve problems such as weak dyeing, increased production costs, and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Aiming at the defects of the dyes in the prior art, the inventors of the present application obtained the asymmetric metal complex black dye suitable for dyeing hydroxyl-containing or amino-containing fiber materials of the present invention through in-depth research. The preparation method of this dyestuff comprises as follows:
[0065]
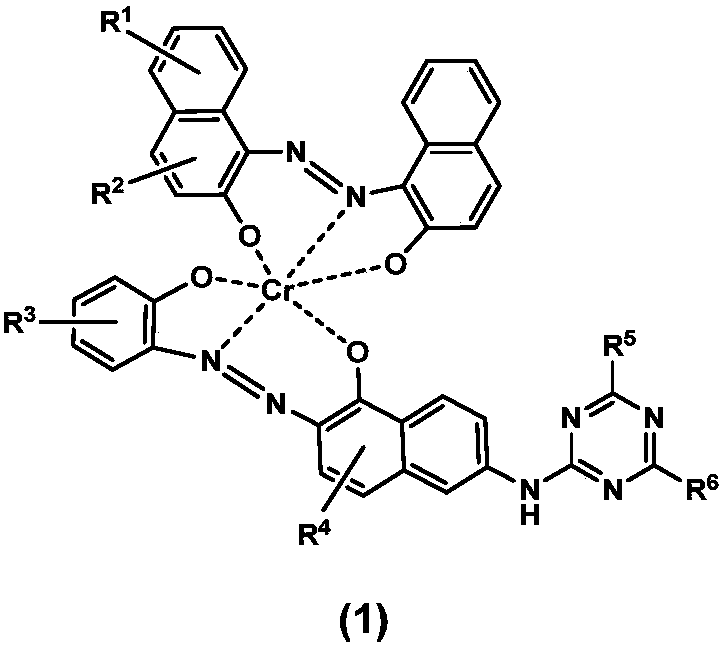
[0066] where R 1 , R 2 , R 3 , R 4 each independently hydrogen, nitro, sulfonic acid group, halogen atom or sulfonamide group, R 5 , R 6 Each is independently a halogen atom, a hydroxyl group, an alkoxy group, an aromatic amine or an aliphatic amine.
[0067] In a preferred embodiment of the present invention, step a) is specifically: dissolving the diazo compound shown in formula (3) in ice water under stirring, adjusting the pH=2-4 with alkali; adding 2-naphthol to the water , use sodium hydroxide and sodium carbonate to adjust pH=9-11, stir and dissolve at 50-90°C, then cool down to 30-40°C; add 2-naphthol solution to the ...
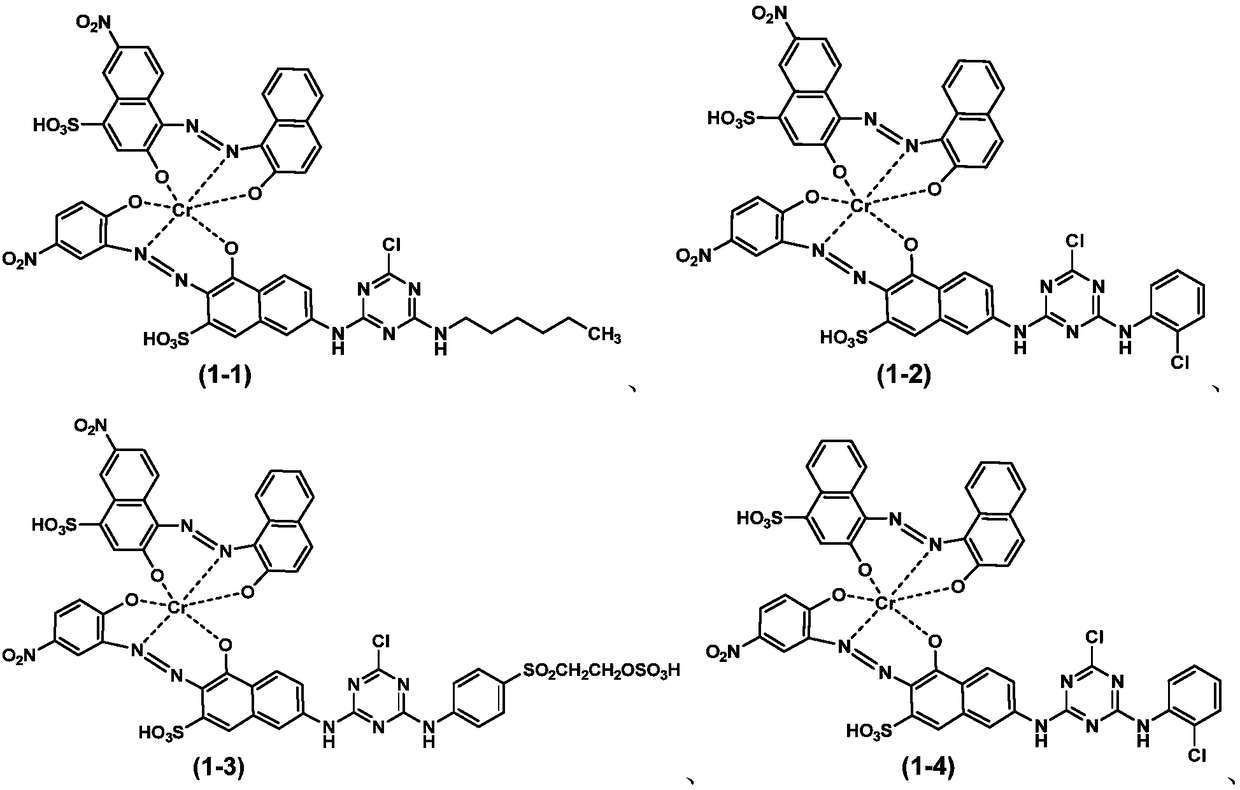
Embodiment 1
[0088] a) Preparation of coupling reactant I:
[0089] Add 29.5 parts of 6-nitro-1,2,4-acid oxygen, as shown in formula (3-1), add 150 parts of ice water to mix and beat, stir and cool down to 5°C, adjust with 30% sodium hydroxide solution The pH of the slurry was 2.0 and stirred until fully dissolved.
[0090] Add 14.5 parts of 2-naphthol to 120 parts of water, add 6 parts of 30% sodium hydroxide solution and 4 parts of sodium carbonate, heat in a hot water bath to 50-60 ° C and stir until completely dissolved, then cool the solution to 35°C.
[0091] Under stirring, quickly pour the above 2-naphthol solution into the 6-nitro-1,2,4-acid oxygen body solution for coupling reaction, adjust the pH of the coupling reaction system to 10.0, keep warm at 40°C, and stir for 5 hours. The progress of the reaction was monitored by liquid chromatography. After completion of the reaction, salting out, filtering, washing with water, and drying to obtain the monoazo compound represented b...
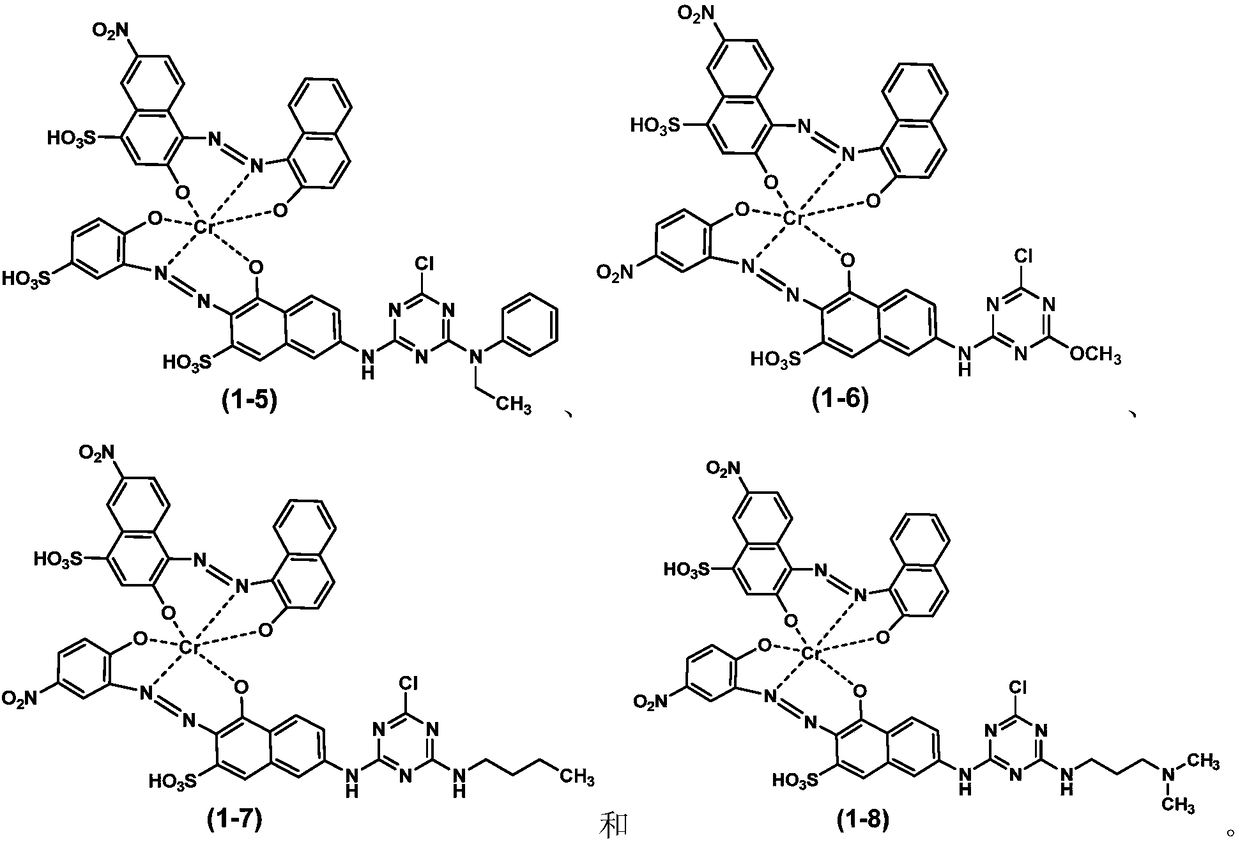
Embodiment 2
[0109] a) Preparation of coupling reactant I:
[0110] Add 29.5 parts of 6-nitro-1,2,4-acid oxygen, as shown in formula (3-1), add 150 parts of ice water to mix and beat, stir and cool down to 5°C, adjust with 30% sodium hydroxide solution pH=4.0, stir until completely dissolved.
[0111] Add 14.5 parts of 2-naphthol to 120 parts of water, add 6 parts of 30% sodium hydroxide solution and 4 parts of sodium carbonate, heat in a hot water bath to 70-80 ° C and stir until completely dissolved, then cool the solution to 35°C.
[0112] Under stirring, quickly pour the 2-naphthol solution into the 6-nitro-1,2,4-acid oxygen body solution for coupling, adjust the coupling pH=10.5, keep warm at 40°C and stir for 6 hours, monitor the reaction with liquid chromatography schedule. After completion of the reaction, salting out, filtering, washing with water, and drying to obtain the monoazo compound represented by formula (2-1).
[0113] b) Preparation of 1:1 chromium complex:
[0114]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com