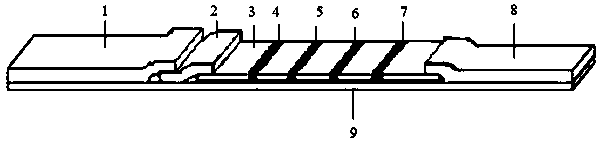
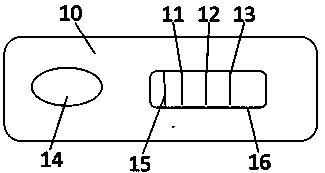
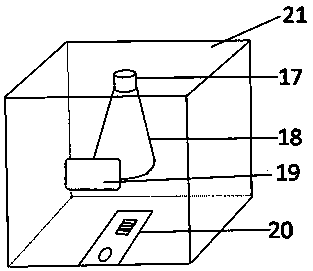
Inflammatory marker POCT joint detection kit
A combined detection and marker technology, applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of limited application, achieve the effect of simple detection operation, reduce pain and the risk of cross-infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Preparation of detection card
[0032] 1. Preparation of CRP and SAA antibody-fluorescent microsphere complexes
[0033] Take 10 μL of Eu-carboxyl fluorescent microspheres and add 400 μL of 0.05M boric acid buffer solution with pH=7.6, mix well; add 40 μL of 1 mg / ml EDC activator and 10 μL of 1 mg / ml NHS solution, shake and activate on a constant temperature oscillator for 20 minutes; Centrifuge at 5000rpm for 20min, discard the supernatant, add 500μL 0.05M pH=7.6 boric acid buffer to redissolve the precipitate, vortex mix, add 40ug CRP and SAA respectively, vortex mix, put in a shaker and oscillate at low speed for 2h; at 5000rpm Centrifuge for 15 min, discard the supernatant, add 1500 μL reconstitution solution to redissolve, and then block with 10 μL blocking solution (20% BSA) for 30 min. The composition of the reconstitution solution is 0.05M pH 8.0 Tris-HCl, 0.1% PVP K30, 0.5 %BSA, 3% Trehalose, 1% Triton X-100, 0.05% NaN 3 .
[0034] 2. Coating mo...
Embodiment 2
[0044] Example 2 Preparation of Fluorescence Immunochromatography Color Card
[0045] 1. Gradient fluorescent microsphere dilution
[0046] a. Take 10 μL of the microsphere stock solution with a concentration of 10 mg / mL into a centrifuge tube.
[0047] b. Add 0.05M PBS to dilute into microsphere solutions with concentrations of 12.5μg / mL, 6.25μg / mL, and 3.125μg / mL;
[0048] 2. Draw a line
[0049] a. Take the nitrocellulose film, paste it on the PVC board, place it on the corresponding position of the three-dimensional dot film gold spraying instrument, and set it aside;
[0050] b. Clean the two pipelines of the three-dimensional dot film spray gold instrument with pure water for 3 times;
[0051] c. Adjust the position of the pipeline so that the distance between the two pipelines is 6mm;
[0052] d. The scribing parameters are set as: scribing speed 50mm / s, scribing volume 1μL / cm;
[0053] e. Inhale the prepared microsphere solutions of different concentrations into t...
Embodiment 3
[0063] The detection limit of the SAA and CRP antibody on the detection card of the present invention is determined
[0064] Sample processing: collect blood with the sample collection device provided in this kit.
[0065] Preparation of the test card: the test card prepared in Example 1 was placed at room temperature for 15 minutes before use.
[0066] Dilute SAA and CRP antigens to 2mg / L, 4mg / L, 8mg / L, 16mg / L, 32mg / L, 64mg / L, 128mg / L,
[0067] Use the combined detection SAA and CRP fluorescent immunochromatography kit set established by the present invention for detection, and put it in the detection device to interpret the result after adding the sample for 5 minutes. The results are shown in Table 1:
[0068] Table 1 The detection limit determination results of SAA and CRP antibodies
[0069]
[0070] It can be seen from Table 1 that the test card detects serial samples diluted with serum amyloid A (SAA) and C-reactive protein (CRP) antigens. From the test results, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com