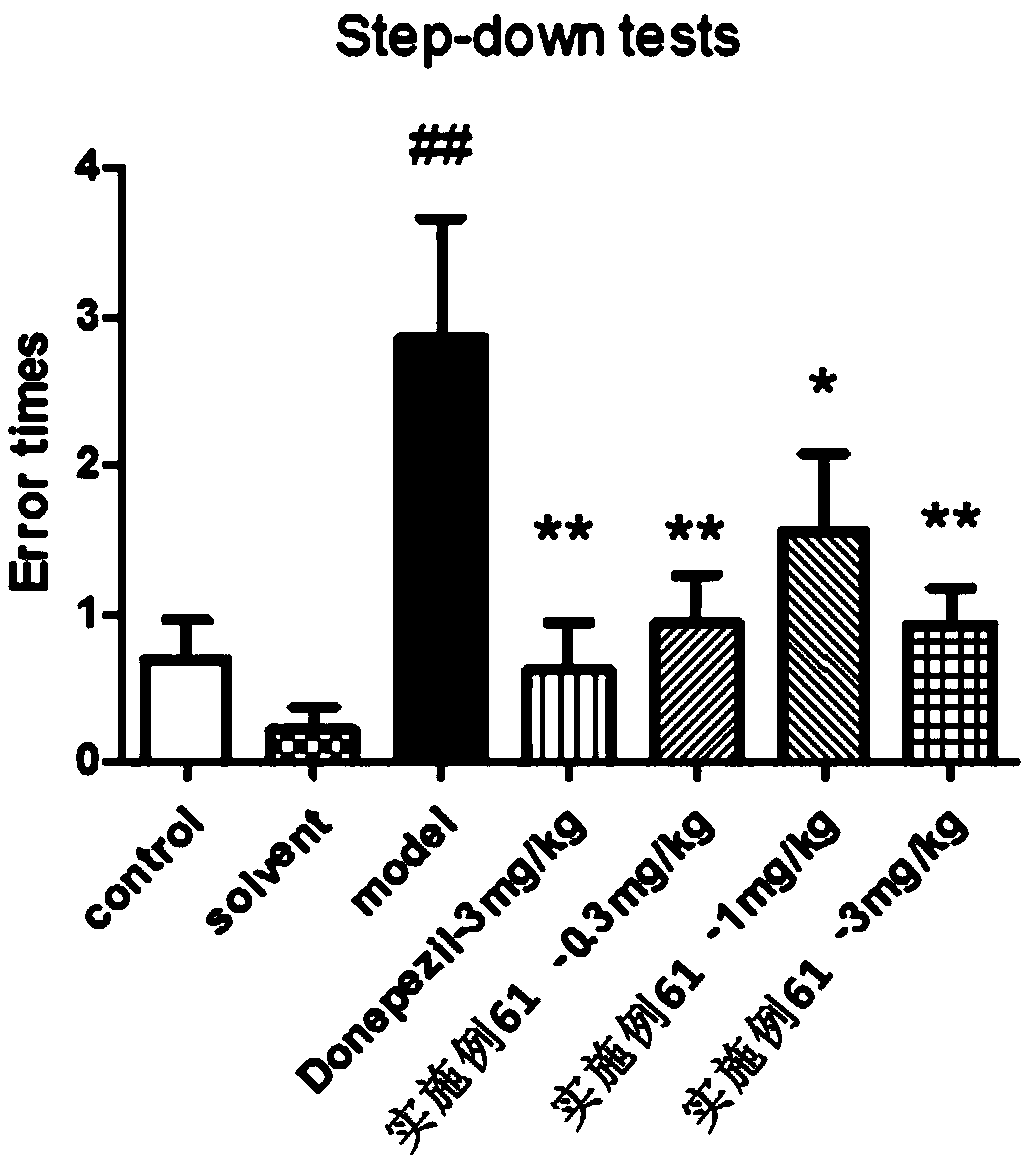
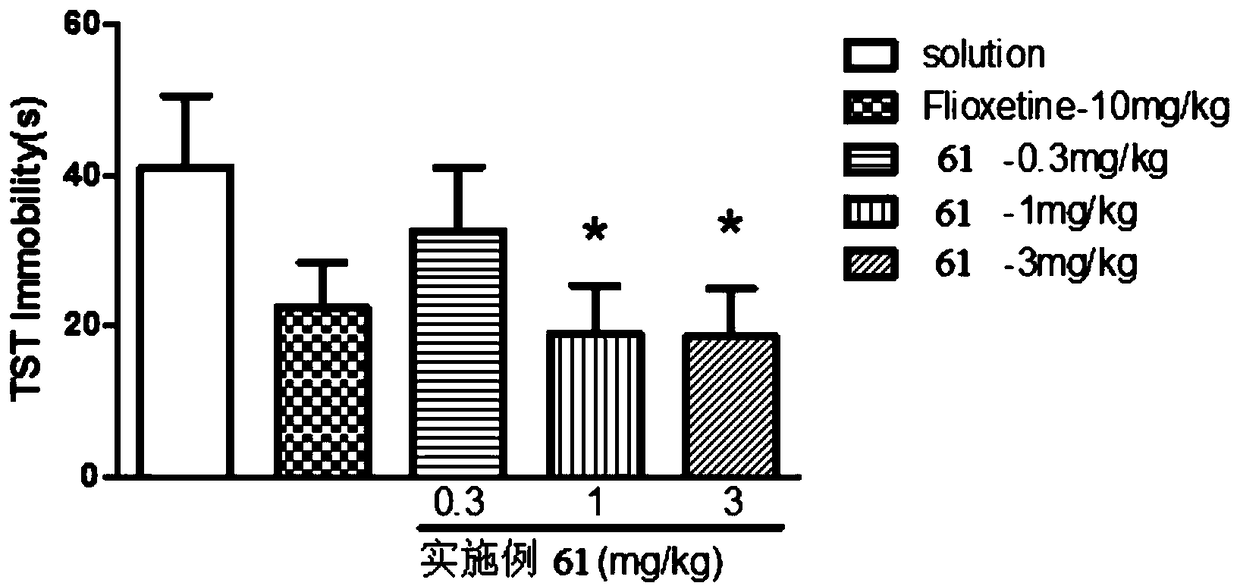
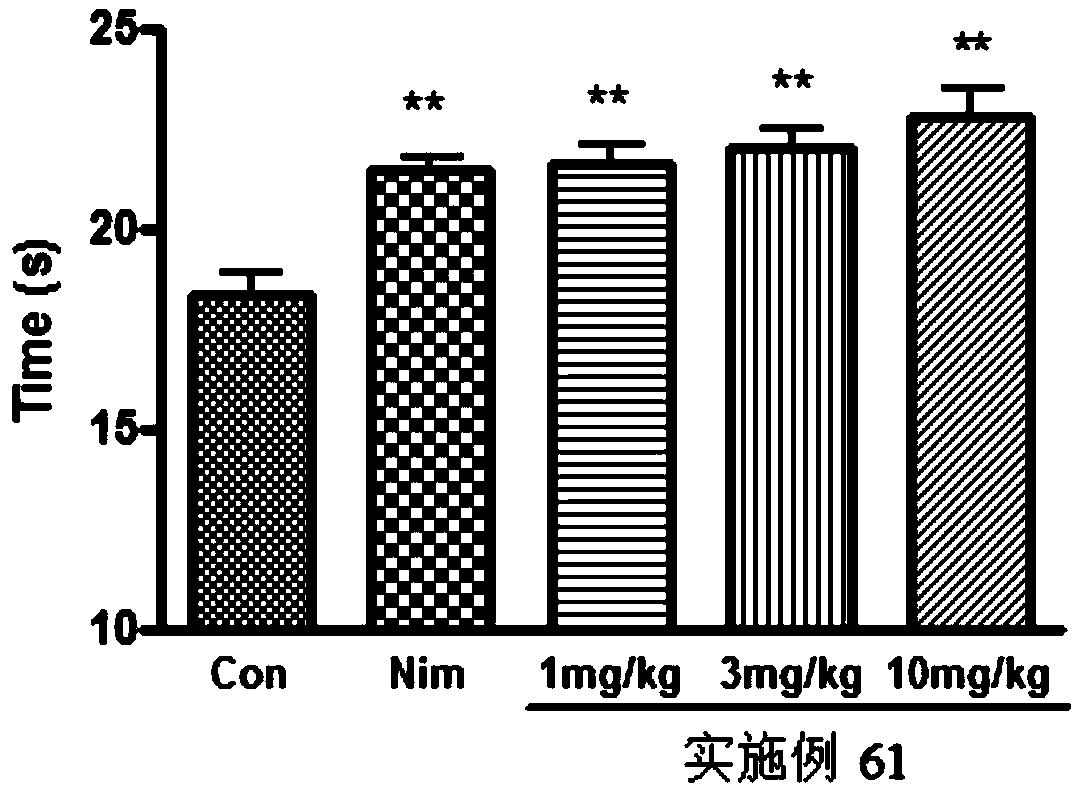
Aromatic amide as kv2.1 inhibitor and preparation method, pharmaceutical composition, and use thereof
A technology for compounds and medicinal salts, which can be applied in the fields of drug combination, preparation of carboxylic acid amides, active ingredients of amides, etc., can solve the problems of poor selectivity, limited application, limited sources of polypeptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0411] Preparation of intermediates
[0412] (1) 2-(dimethylamino)-5-nitrobenzoic acid
[0413]
[0414] Take 2-chloro-5-nitrobenzoic acid (2.0g, 10.00mmol), add dimethylamine (40% aqueous solution) (20mL), heat to 65°C, after 8h the reaction of raw materials is complete, add dilute acetic acid solution dropwise, Adjust the pH to acidic, extract with EA (30mL×5), combine and concentrate, and dry to obtain 1.6g of yellow solid, 76.7%.
[0415] 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 8.92 (d, J = 2.8Hz, 1H), 8.33 (dd, J = 9.2, 2.8Hz, 1H), 7.36 (d, J = 9.2Hz, 1H), 2.96 (s, 6H) ;ESI-MS m / z:209.06[M-H] - .
[0416] (2) Preparation of 2-ethoxy-5-benzoic acid
[0417]
[0418] a) Methyl 2-hydroxy-5-nitrobenzoate
[0419]
[0420] 2-Hydroxy-5-nitrobenzoic acid (6 g, 32.7 mmol) was dissolved in anhydrous methanol (50 mL), and SOCl was added slowly under stirring 2 The solution was reacted at 60°C for 10 h, cooled, filtered, and the filter cake was washed with ice methanol to...
Embodiment 1
[0493] 2-(Dimethylamino)-5-isobutyrylamino-N-(pyridin-2-ylmethyl)benzamide
[0494]
[0495] a) 2-(dimethylamino)-5-nitro-N-(pyridin-2-ylmethyl)benzamide
[0496] Dissolve 2-(dimethylamino)-5-nitrobenzoic acid (200mg, 0.95mmol) in DMF (15mL), add DIEA (185mg, 1.43mmol) and HATU (542mg, 1.43mmol) successively, and add after 20min 2-Aminomethylpyridine (93mg, 0.86mmol), stirred overnight at room temperature. Concentrate, add EA (20mL) to dilute, wash with HCl (0.5N) (10mL), saturate with NaHCO 3 (10mL) wash, water (10mL) wash, anhydrous Na 2 SO 4 It was dried and purified by column chromatography (ethyl acetate-petroleum ether, volume ratio 2:3) to obtain 248 mg of yellow solid, yield 95.0%, melting point: 140-142°C.
[0497] 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 8.58-8.55 (m, 2H), 8.27 (s, 1H), 8.17 (dd, J = 9.2, 1.6Hz, 1H), 7.71 (t, J = 7.6Hz, 1H), 7.36 ( d,J=7.6Hz,1H),7.23(d,J=6.0Hz,1H),6.96(d,J=9.2Hz,1H),4.78(d,J=4.8Hz,2H),2.95(s, 6H); HR-MS (ESI): m / z, calcd. For C ...
Embodiment 2
[0502] 2-(Dimethylamino)-5-isobutyrylamino-N-(1-(pyridin-2-yl)ethyl)benzamide
[0503]
[0504] a) 2-(Dimethylamino)-5-nitro-N-(1-(pyridin-2-yl)ethyl)benzamide
[0505] Dissolve 2-(dimethylamino)-5-nitrobenzoic acid (356mg, 1.69mmol) in DMF (10mL), add DIEA (696mg, 5.40mmol) and HATU (965mg, 2.54mmol) successively, and stir for 20min Then 1-(pyridin-2-yl)ethylamine·hydrochloride (300mg, 1.54mmol) was added and stirred overnight at room temperature. Concentrate, add EA (20mL) to dilute, wash with saturated ammonium chloride solution (10mL), wash with saturated NaHCO 3 (10mL) wash, water (10mL) wash, anhydrous Na 2 SO 4 It was dried and purified by column chromatography (ethyl acetate-petroleum ether, volume ratio 1:2) to obtain 247 mg of yellow solid, yield 51.1%, melting point: 158-160°C.
[0506] 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 8.56 (d, J = 4.8Hz, 1H), 8.49 (d, J = 2.8Hz, 1H), 8.35 (d, J = 6.8Hz, 1H), 8.14 (dd, J = 9.2, 2.8Hz, 1H), 7.73(t, J=7.6Hz, 1H), 7.35(d, J=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com