Azoxystrobin acetone solvate and preparation method thereof
A technology of acetone solvent and solvate, applied in the field of azoxystrobin acetone solvate and preparation, can solve the problems of difficult filtration, high cost, low efficiency, etc., and achieve the effects of not easy to coalesce, complete crystal habit, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
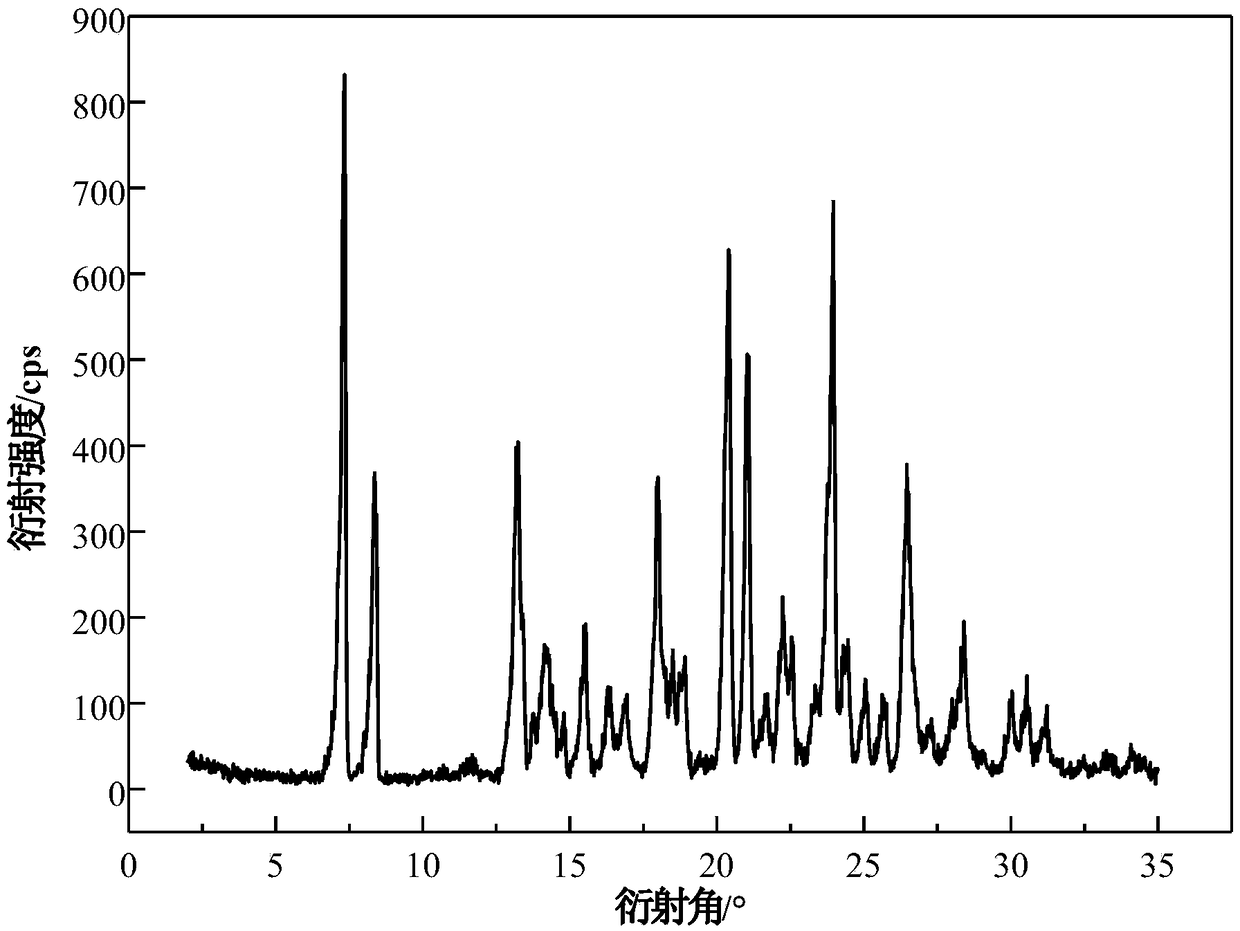
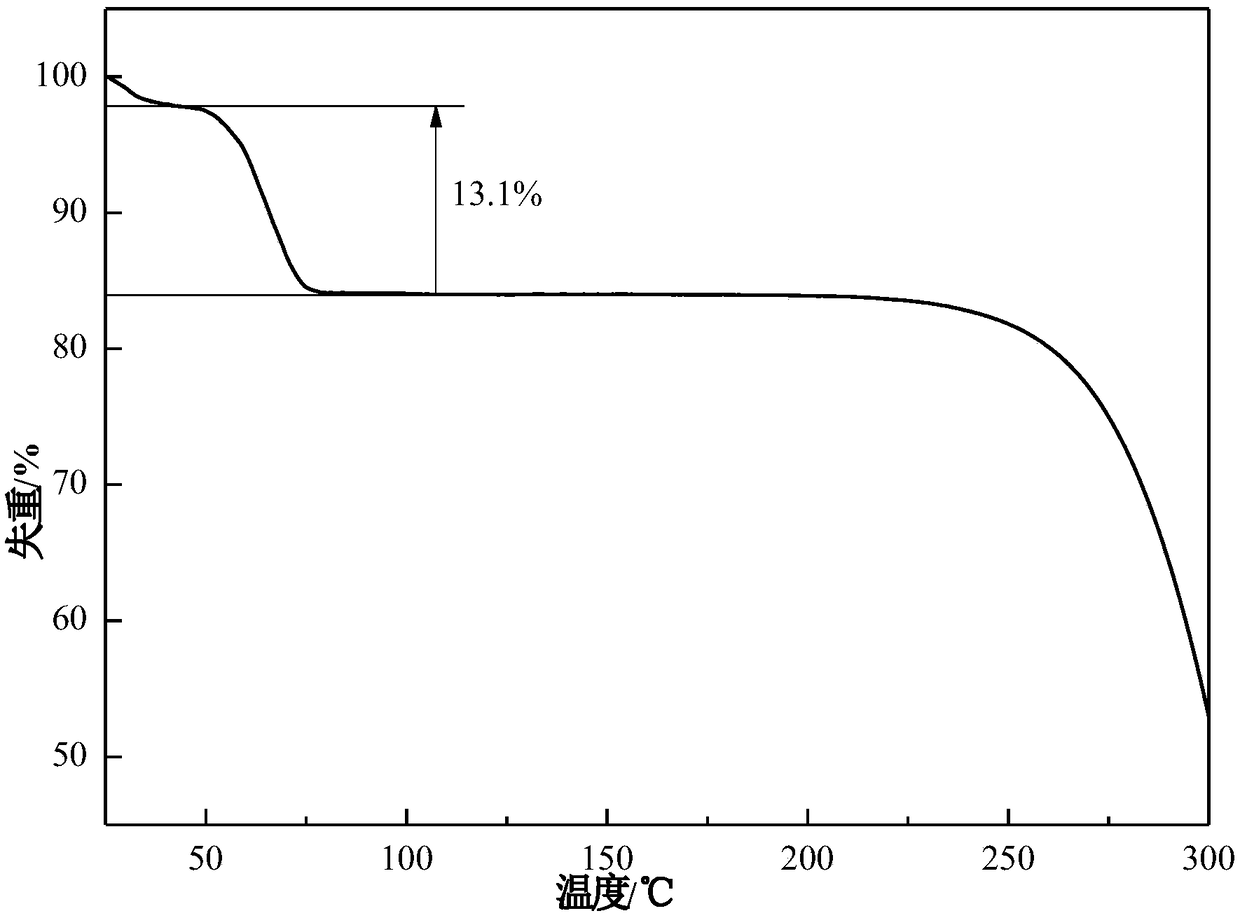
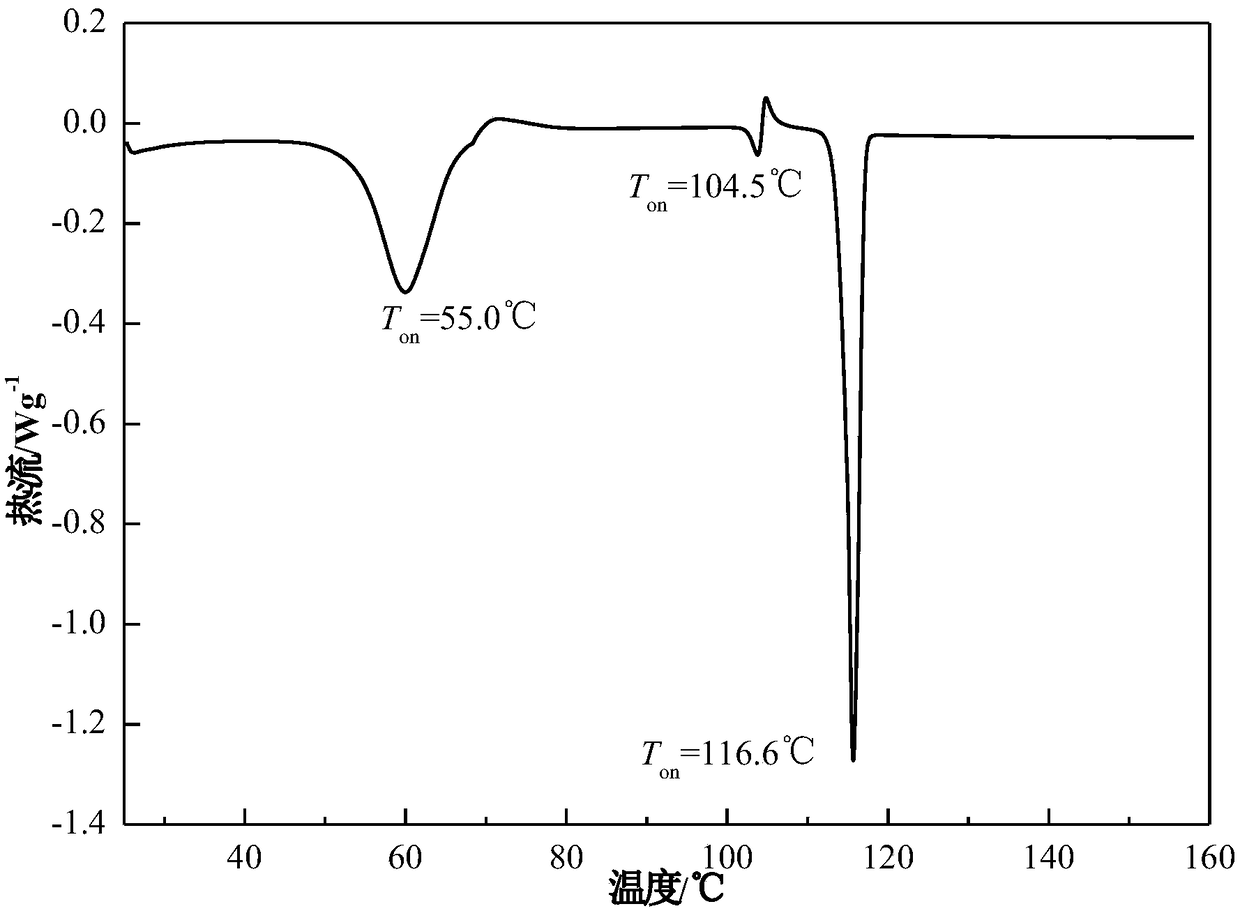
[0038] Take 20g of acetone into the crystallizer, stir at 20°C, add 5g of azoxystrobin raw material into the crystallizer in 2 batches for suspension crystallization, after adding the raw material, continue to stir the solution for 1h, filter the solid, and dry to obtain the product. The X-ray powder diffraction pattern of the product is as follows figure 1 , at 7.32±0.20°, 8.42±0.20°, 13.26±0.20°, 14.16±0.20°, 18.04±0.20°, 18.52±0.20°, 18.90±0.20°, 20.40±0.20°, 21.04±0.20°, 22.36±0.20 °, 23.96±0.20°, 24.36±0.20°, 26.46±0.20°, 28.42±0.20°, 30.06±0.20°, etc. have characteristic peaks, of which 7.32±0.20° is the initial peak, and the relative intensity is 100%. Its TGA results are as follows figure 2 , there will be a weight loss of 13.1%±0.5% before heating to 80°C. Its DSC results are as follows image 3 , there is an endothermic peak at 55.0±5°C, an endothermic and exothermic peak at 104.5±5°C, and a characteristic melting peak at 116.6±5°C. Its IR result is as Figure ...
Embodiment 2
[0040] Take 25g of acetone into the crystallizer, stir at 25°C, add 6g of azoxystrobin raw material into the crystallizer in 3 batches for suspension crystallization, after adding the raw material, continue to stir the solution for 1.5h, filter the solid, and dry to obtain the product. The X-ray powder diffraction pattern of the product is as follows figure 1 . Its TGA and DSC curves are as figure 2 , image 3 . Its infrared spectrum (IR) is as Figure 4 . crystal shape and Figure 5 Similar to a rod. It shows that what is obtained is azoxystrobin acetone solvate. Its volume average particle size is 40μm, its angle of repose is 26°, it does not coalesce and has good fluidity. The XRPD of the azoxystrobin raw material crystal form is as follows: Figure 7 It is Form A.
Embodiment 3
[0042] Add 30g of acetone into the crystallizer, stir at 30°C, add 8g of azoxystrobin raw material into the crystallizer in 2 batches, and carry out suspension crystallization. After adding the raw materials, continue to stir the solution for 2h, filter the solid, and dry to obtain the product. The X-ray powder diffraction pattern of the product is as follows figure 1 . Its TGA and DSC curves are as figure 2 , image 3 . Its infrared spectrum (IR) is as Figure 4 . crystal shape and Figure 5 Similar to a rod. It shows that what is obtained is azoxystrobin acetone solvate. Its volume average particle size is 40μm, its angle of repose is 22°, it does not coalesce and has good fluidity. The XRPD of the azoxystrobin raw material crystal form is as follows: Figure 7 It is Form A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com