A kind of diamine monomer containing pyrazine structure and its preparation method and a kind of polyimide containing pyrazine structure and its preparation method
A technology of diamine monomer and polyimide, which is applied in the field of polyimide containing pyrazine structure and its preparation, can solve problems such as the difficulty of applying transparent optical materials, and achieve improved electron transport properties and good dissolution properties, reducing the effect of close packing of molecular chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides the preparation method of the diamine monomer containing pyrazine structure, comprising the following steps:
[0045] Mix chloropyrazine, nitrophenol and alkaline ionic liquid to undergo nucleophilic substitution reaction to obtain dinitro compounds;
[0046] Mixing the dinitro compound, phosphotungstic acid, zinc powder and an organic solvent for a reduction reaction to obtain a diamine monomer containing a pyrazine structure;
[0047] Wherein, the chloropyrazine is
[0048] The nitrophenol is
[0049] In the present invention, unless otherwise specified, all raw materials are commercially available products well known to those skilled in the art.
[0050] The invention mixes chloropyrazine, nitrophenol and alkaline ionic liquid to undergo nucleophilic substitution reaction to obtain dinitro compound. In the present invention, the chloropyrazine is preferably 2,6-dichloropyrazine, 2,2'-dichlorobipyrazine, 2,7-dichloroquino...
Embodiment 1
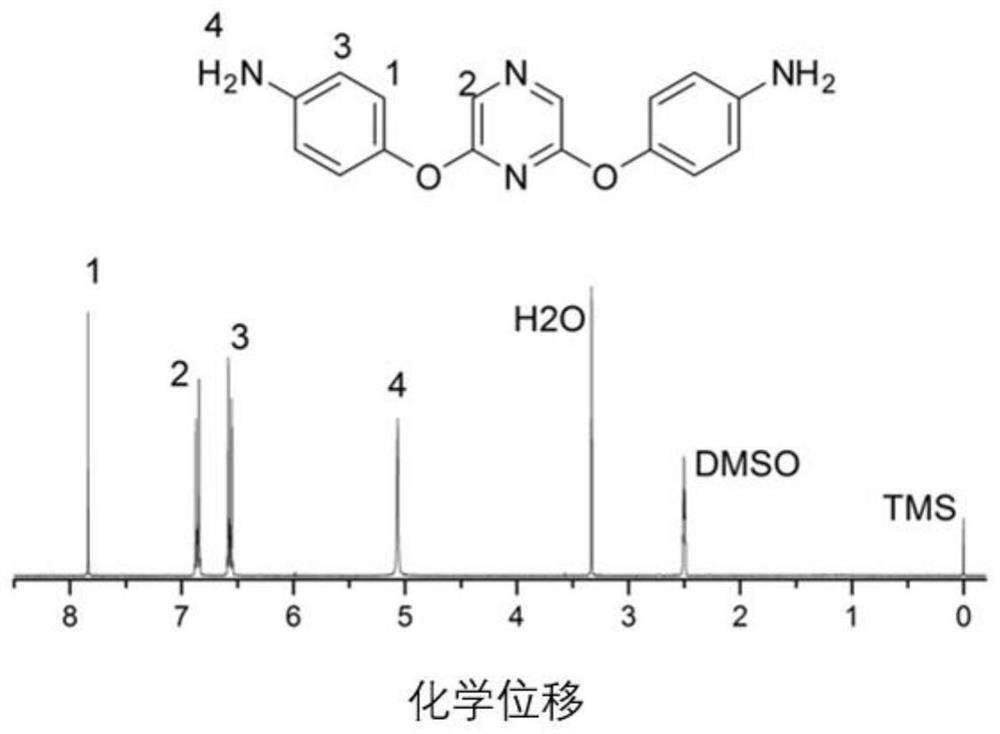
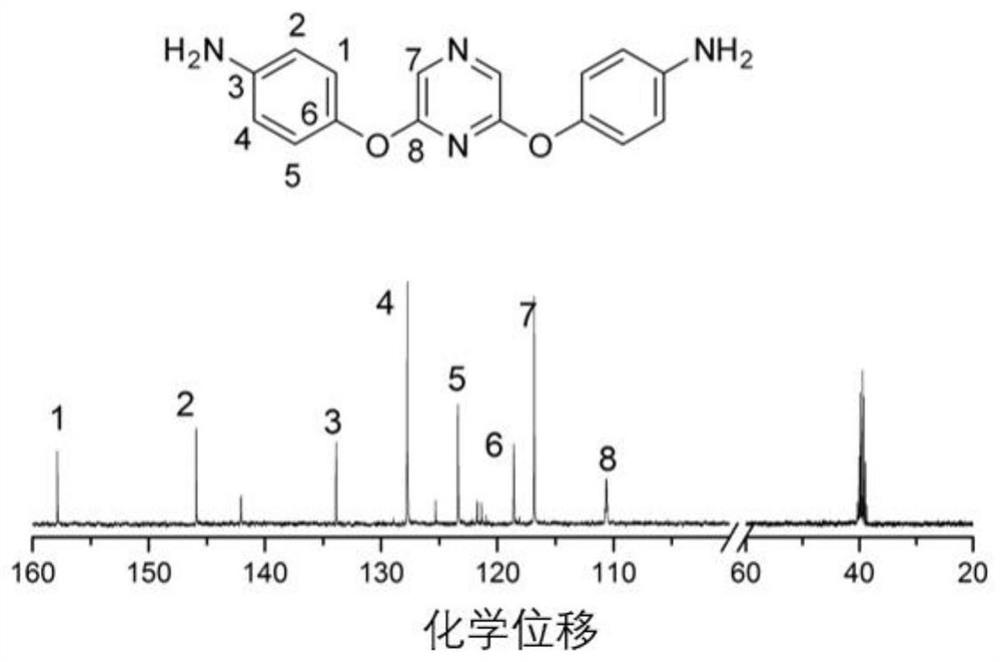
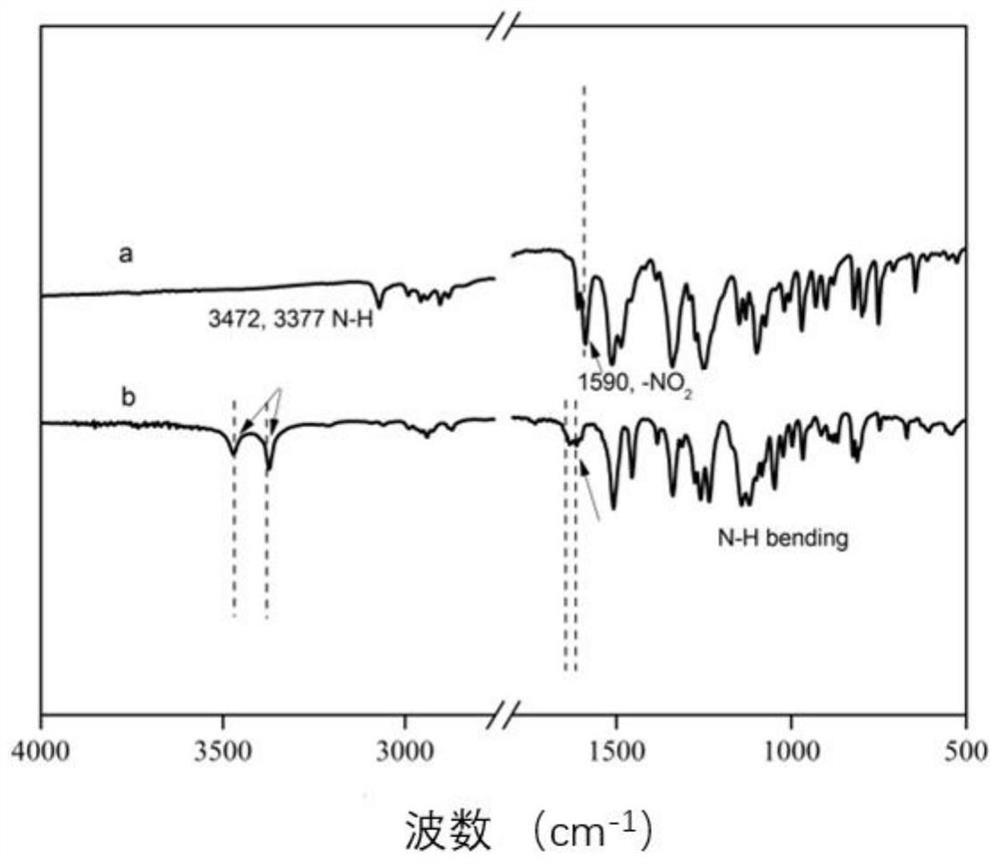
[0095] The preparation of 2,6-bis (4-aminophenoxy) pyrazine, its structural formula is as follows:
[0096]
[0097] At normal temperature and pressure, 100mmol 2,6-dichloropyrazine, 210mmol p-nitrophenol, and 2200mmol basic ionic liquid [bmin]OH were transferred to a three-necked flask, and reacted for 5 hours under stirring and nitrogen protection. get the product system;
[0098]Pour the obtained product system into deionized water, recover the basic ionic liquid [bmin]OH, filter the precipitated solid with suction, wash 3 times with deionized water, dry at 80°C for 12 hours, and recrystallize with methanol. 2,6-bis(4-nitrophenoxy)pyrazine was obtained with a yield of 85%.
[0099] Dissolve 50mmol of 2,6-bis(4-nitrophenoxy)pyrazine in 100mmol of absolute ethanol, add 1.25mmol of phosphotungstic acid and 250mmol of zinc powder under nitrogen protection, transfer the mixed solution to a high-pressure reactor, and React for 3 hours at 0.3Mpa and 180°C; filter to remove un...
Embodiment 2
[0103] The preparation of 2,2'-bis(2-methyl-4-aminophenoxy)bipyrazine, its structural formula is as follows:
[0104]
[0105] At normal temperature and pressure, 100mmol 2,2'-dichlorobipyrazine, 220mmol 2-hydroxy-5-nitrotoluene, and 2400mmol basic ionic liquid [bmin]OH were transferred to a three-necked flask, under stirring and nitrogen protection. Under the condition of reaction for 8 hours, the product system was obtained;
[0106] Pour the obtained product system into deionized water, recover the basic ionic liquid [bmin]OH, filter the precipitated solid with suction, wash 5 times with deionized water, dry at 80°C for 12 hours, and recrystallize with methanol. 2,2'-bis(2-methyl-4-nitrophenoxy)bipyrazine was obtained with a yield of 85%.
[0107] Dissolve 50mmol of 2,2'-bis(2-methyl-4-nitrophenoxy)bipyrazine in 200mmol of anhydrous methanol, add 1.25mmol of phosphotungstic acid and 500mmol of zinc powder under nitrogen protection, and dissolve the mixture Transfer to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com