A kind of preparation method of prazogamicin antibiotic
A technology of antibiotics and sisomicin, which is applied in the fields of medicinal chemistry and pharmaceutical engineering, can solve problems such as difficult industrial production, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0032] A typical implementation of the present application provides
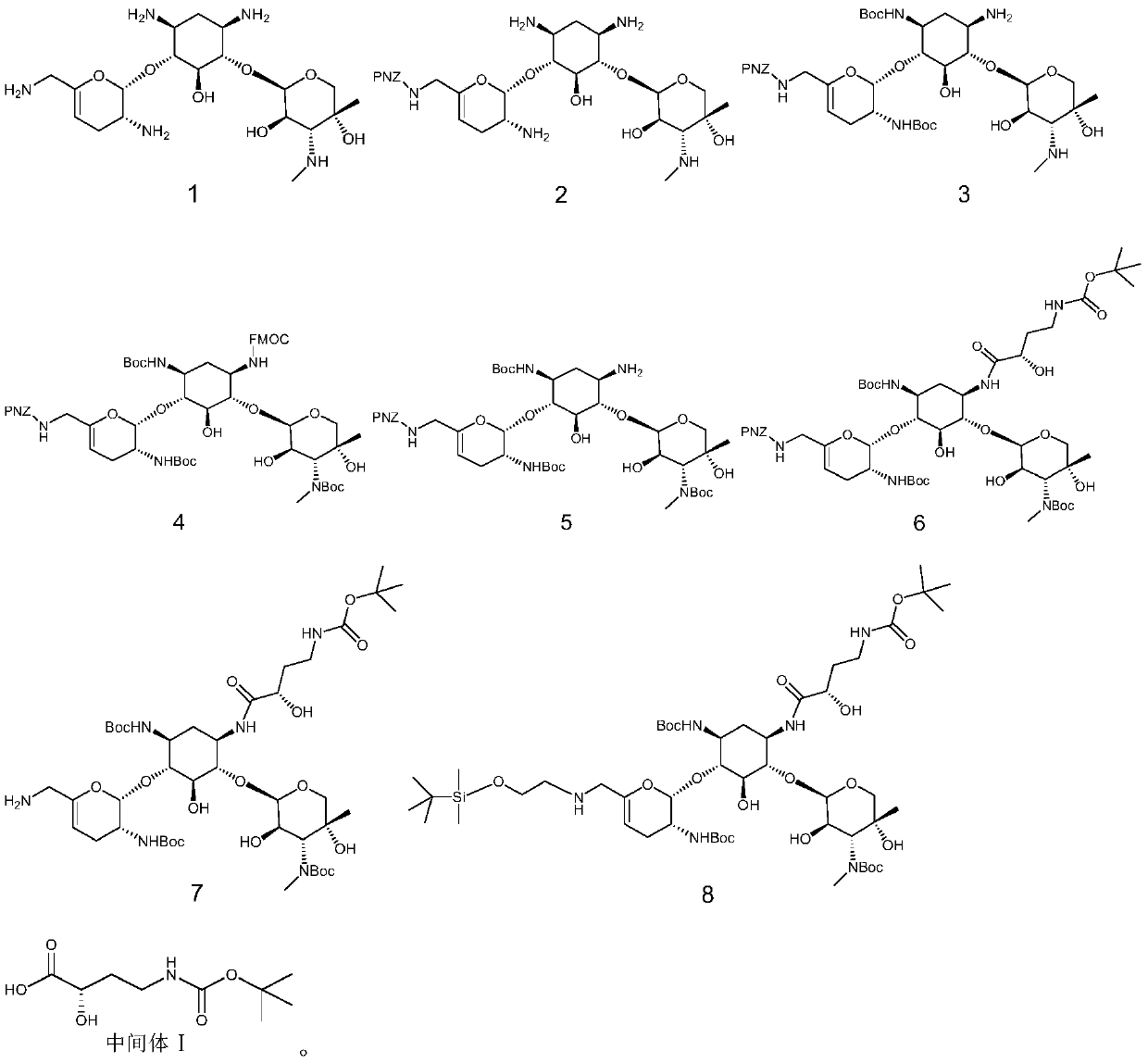
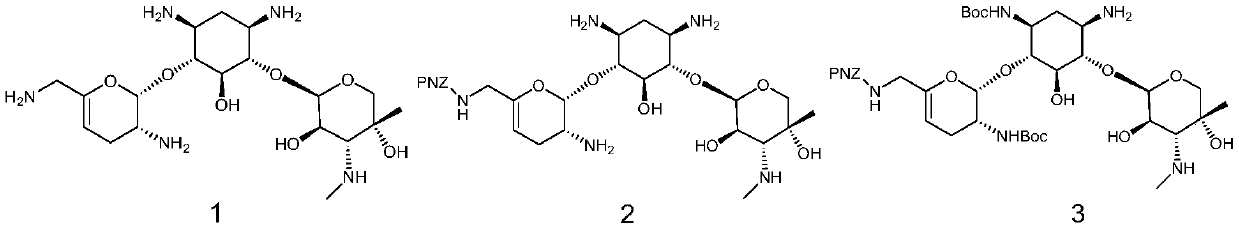
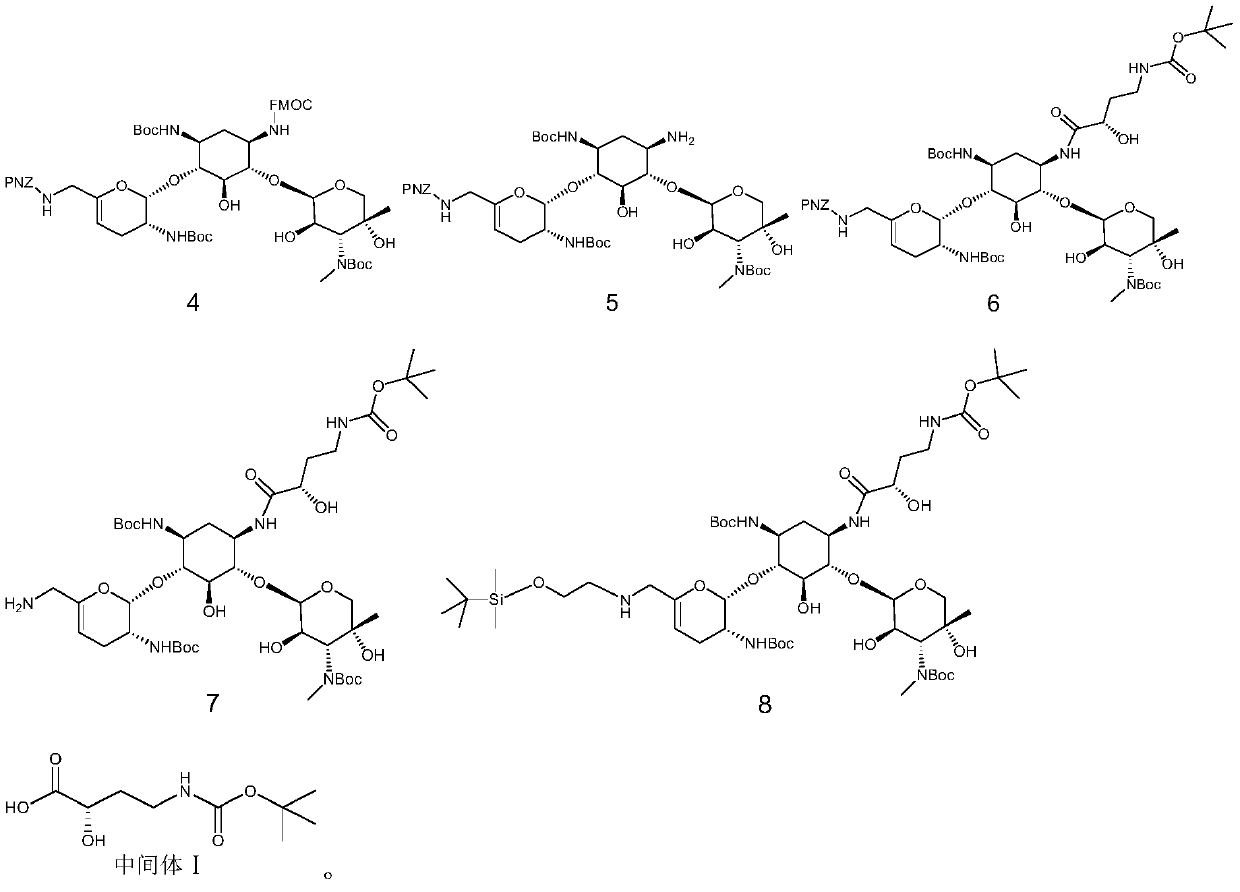
[0033] A kind of preparation method of prazogamicin antibiotic, its step is:
[0034] (1) Sisomicin is removed from the sulfuric acid molecule to obtain the free base shown in formula 1; (2) under the catalysis of Lewis acid, the free base shown in formula 1 carries out nucleophilic substitution reaction with the compound containing PNZ protecting group to obtain The compound shown in formula 2; (3) under the catalysis of Lewis acid and organic base, the compound shown in formula 2 carries out nucleophilic substitution reaction with the compound containing Boc protecting group to obtain the compound shown in formula 3; (4) the compound shown in formula 3 The shown compound first carries out nucleophilic substitution reaction with the compound containing the FOMC protecting group, and then under the action of base catalysis, carries out the nucleophilic substitution reaction with the compound containing the B...
Embodiment 1
[0080] Proceed as follows:
[0081] (1) Synthesis of compound shown in formula 1
[0082]
[0083] Add NaOH (14.52 g, 363 mmol) to a stirred methanol solution (600 mL) of sisomicin sulfate (50.06 g, 72.59 mmol) at room temperature, stir at room temperature for 5 h, filter, concentrate, and dry in vacuo to obtain formula The compound shown in 1 (31.53g, 70.45mmol), yield 97%.
[0084] (2) Synthesis of compound shown in formula 2
[0085]
[0086] In the stirred MeOH (600mL) solution of the compound shown in Formula 1 (33.64g, 70.45mmol) was added Zn(OAc) 2 (38.78 g, 211.36 mmol), and the reaction mixture was stirred for 2 hours until all the zinc went into solution (one spot by TLC). Then a solution of PNZ-ONB (22.26 g, 62.12 mmol) in DCM (400 mL) was added dropwise within 2 hours, and reacted at room temperature for 10 h. After the completion of the reaction monitored by TCL, the reactant was concentrated to dryness to obtain a crude product. The crude product was s...
Embodiment 2
[0126] Embodiment 2: This embodiment is a further enlargement of Embodiment 1.
[0127] (1) Synthesis of compound shown in formula 1
[0128] To a stirred solution of sisomicin sulfate (200 g, 0.29 mol) in methanol (2 L) was added NaOH (57.99 g, 1.45 mol) at room temperature, and the reaction mixture was stirred for 5 h. Concentrate by filtration and dry in vacuo to obtain the compound represented by Formula 1 (125.31 g, 0.28 mol) with a yield of 97%.
[0129] (2) Synthesis of compound shown in formula 2
[0130] Add Zn(OAc) in the MeOH (1.5L) solution of compound (125.31g, 0.28mol) shown in the formula 1 that stirs 2 (154.12 g, 0.84 mol) and the reaction mixture was stirred for 2 hours until all the zinc went into solution (one spot by TLC). Then a solution of PNZ-ONB (85.99 g, 0.24 mol) in DCM (1 L) was added dropwise, and the reaction was stirred at room temperature for 10 h (reaction monitored by TCL). The reaction mixture was concentrated to dryness to give crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com