Kit for detecting hepatocellular carcinoma (HCC)
A hepatocellular carcinoma and reagent kit technology, applied in the field of medical testing, can solve the problems of unsatisfactory treatment effect, limited clinical application, and low sensitivity of blood detection method, and achieve low cost, less blood consumption, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Extraction of ctDNA
[0022] Blood samples were collected in StreckBCT tubes, stored at 4 °C, and ctDNA was extracted within 24 h of collection. Using the Circulating Nucleic Acid kit of QIAamp Company, the ctDNA in plasma was extracted according to the operation steps in the manual.
[0023] 2. Detection of ctDNA length
[0024] The length of the extracted ctDNA was checked by agarose gel electrophoresis. The specific steps are:
[0025] 1) Add 1 g of agarose to 100 mL of 1×TAE running buffer and shake well. Heat in the microwave until the agarose is completely dissolved. Cool to 60°C, add 100 μL of 0.5 mg / mL EB, and shake well.
[0026] 2) Seal both ends of the glue making plate with tape, insert a comb, pour the dissolved agarose, and cool at room temperature to solidify.
[0027] 3) After fully solidified, tear off the tapes at both ends, put the gel into the electrophoresis tank, add 1×TAE electrophoresis buffer until the liquid surface covers the gel by 1-...
Embodiment 2
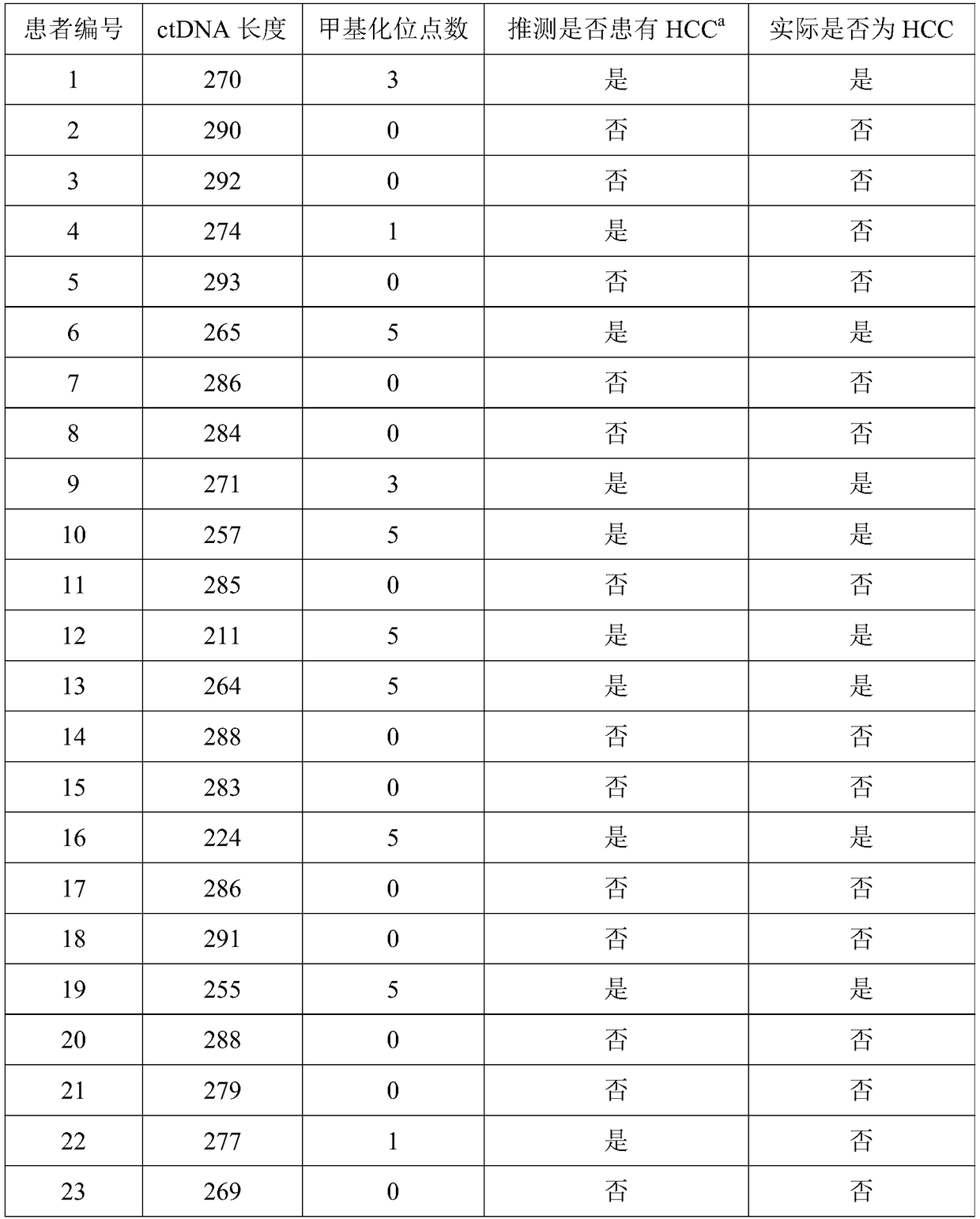
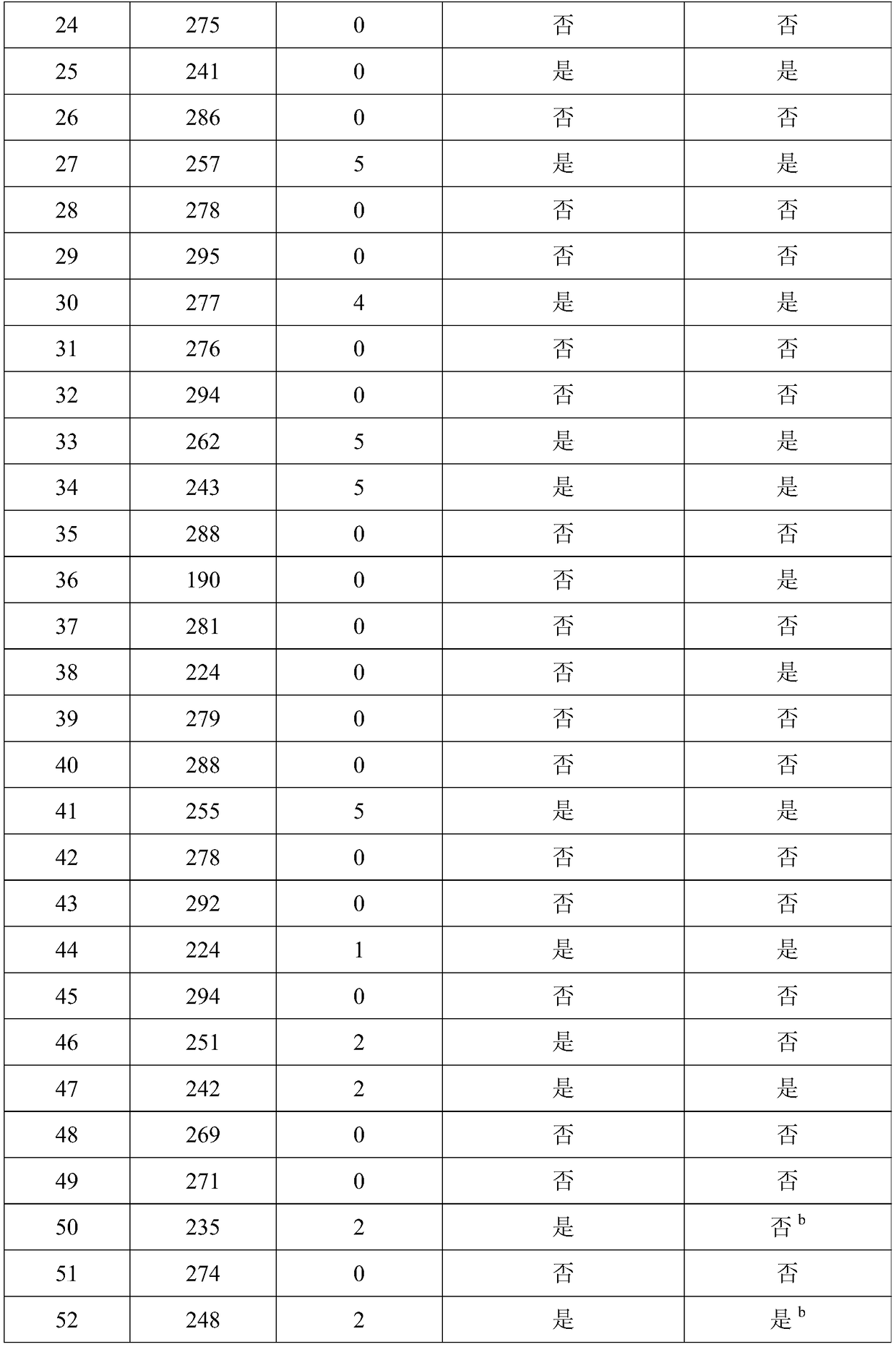
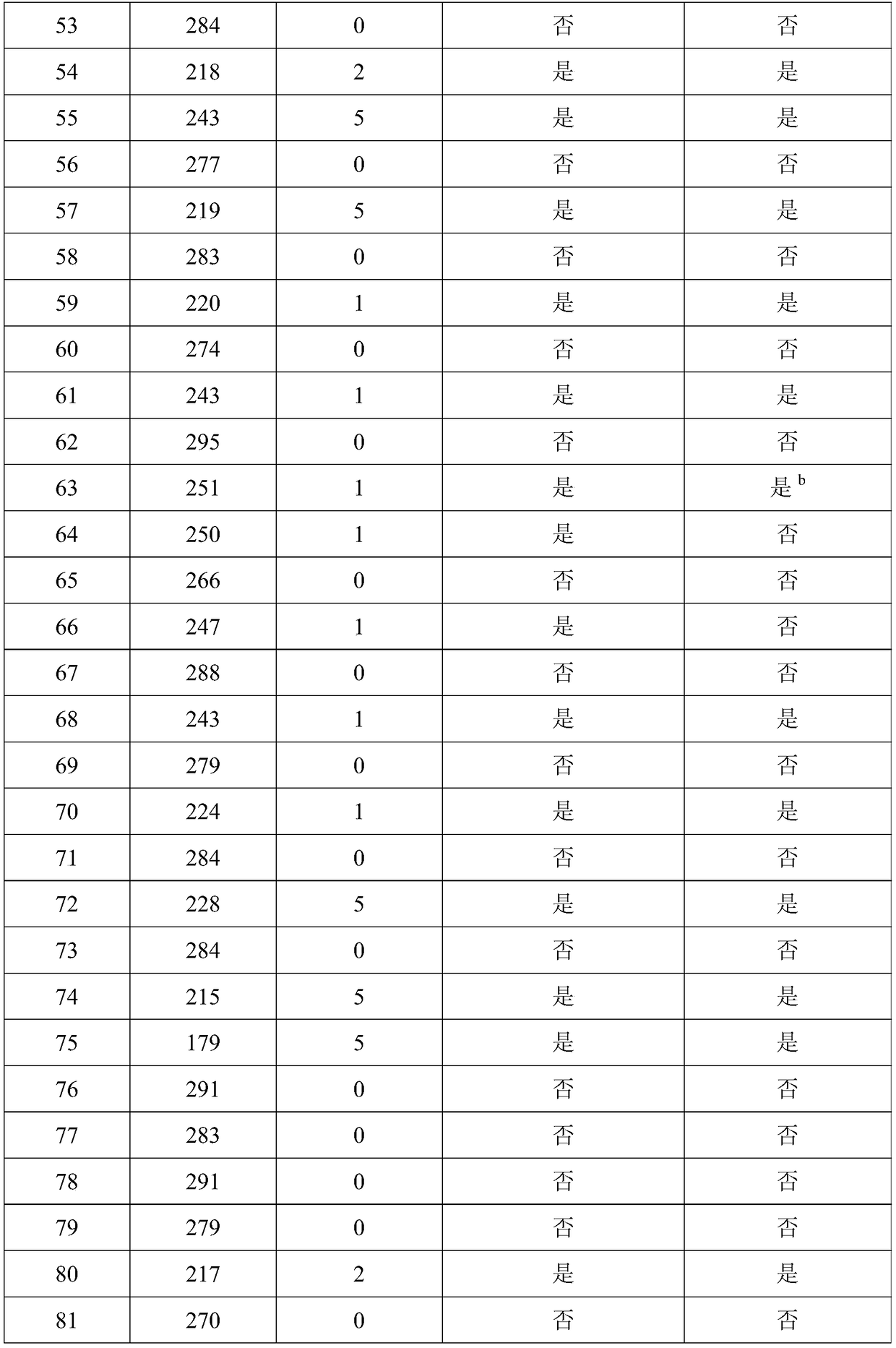
[0048] Double-blind testing was performed as in Example 1.
[0049] Subjects: A total of 202 subjects were enrolled from September 2017 to April 2018. The enrolled patients met the diagnosis of primary liver cancer in the "China Common Malignant Tumor Diagnosis and Treatment Standards"; the age range was 18-70 years old (including 18 years old, 70 years old); there is a clear measurable tumor; no tumor therapy such as radiotherapy and chemotherapy and immunotherapy are used in the first month of enrollment; the quality of life is greater than 60 points; the expected survival period of the subjects is greater than 3 months; understood and signed the informed consent.
[0050] According to the "Guiding Principles of Clinical Research on New Chinese Medicines" according to TCM syndrome differentiation, primary liver cancer belongs to deficiency of both spleen and kidney, and internal resistance of blood stasis and toxin. Symptoms include abdominal distention and fullness, lack o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com