Benazepril hydrochloride chewable tablet for pet and preparation method of benazepril hydrochloride chewable tablet
A technology of benazepril hydrochloride and chewable tablets, which is applied in the field of pet benazepril hydrochloride chewable tablets and its preparation, can solve the problems of increased difficulty in disintegration and dissolution, and solve the problems of difficult disintegration and dissolution, combination Reasonable portion ratio and the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
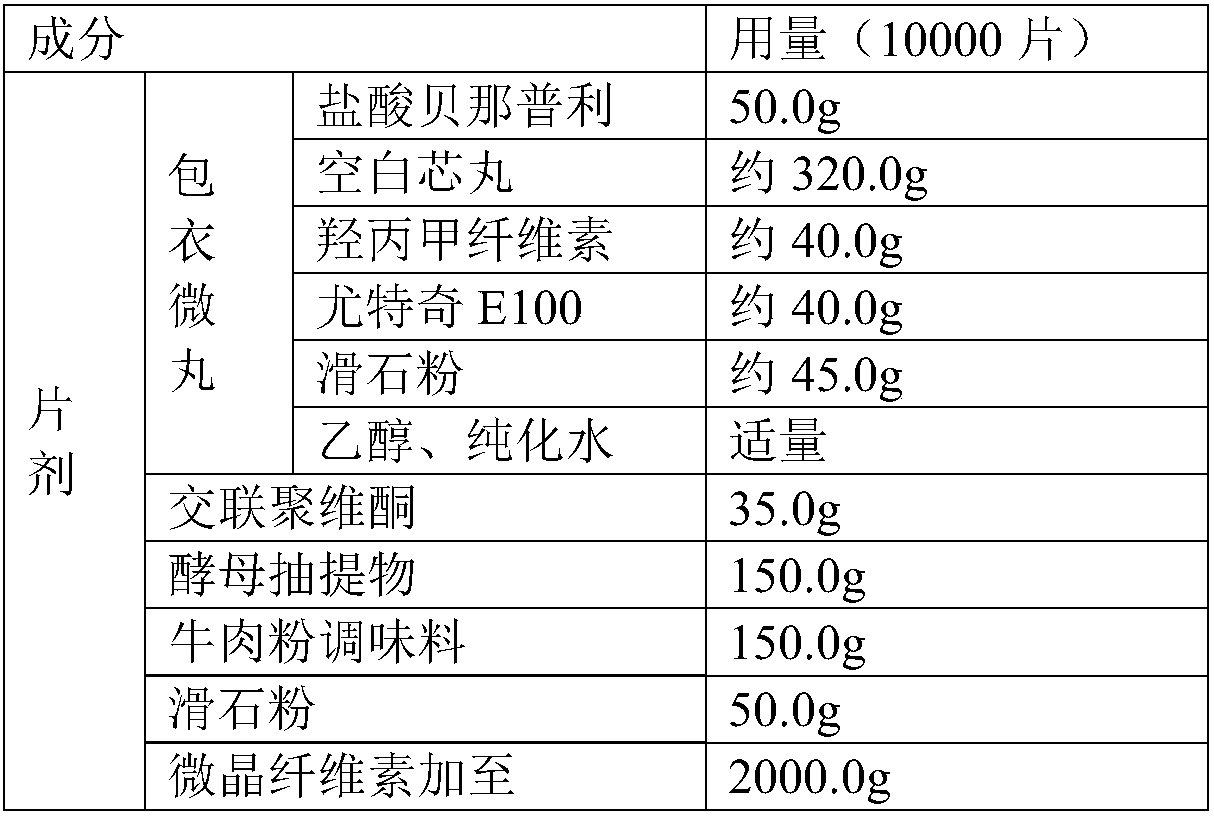
[0039] A pet benazepril hydrochloride chewable tablet, comprising the following raw materials in weight percentage: benazepril hydrochloride 2.5%, binding agent 2%, diluent 56%, disintegrant 1.75%, glidant 2.5%, 15% flavoring agent, 20.25% coating taste-masking material.
[0040] In the present embodiment, the prescription adopted for the pet benazepril hydrochloride chewable tablet is the following table:
[0041]
[0042] In this prescription, the disintegrating agent is crospovidone.
[0043] The adhesive is hypromellose.
[0044] The flavoring agent is selected from yeast extract and beef powder seasoning.
[0045] Glidant selects talcum powder for use.
[0046] The diluent is microcrystalline cellulose.
[0047] Taste-masking materials for coating include blank core pellets, Eudragit E100 and talcum powder.
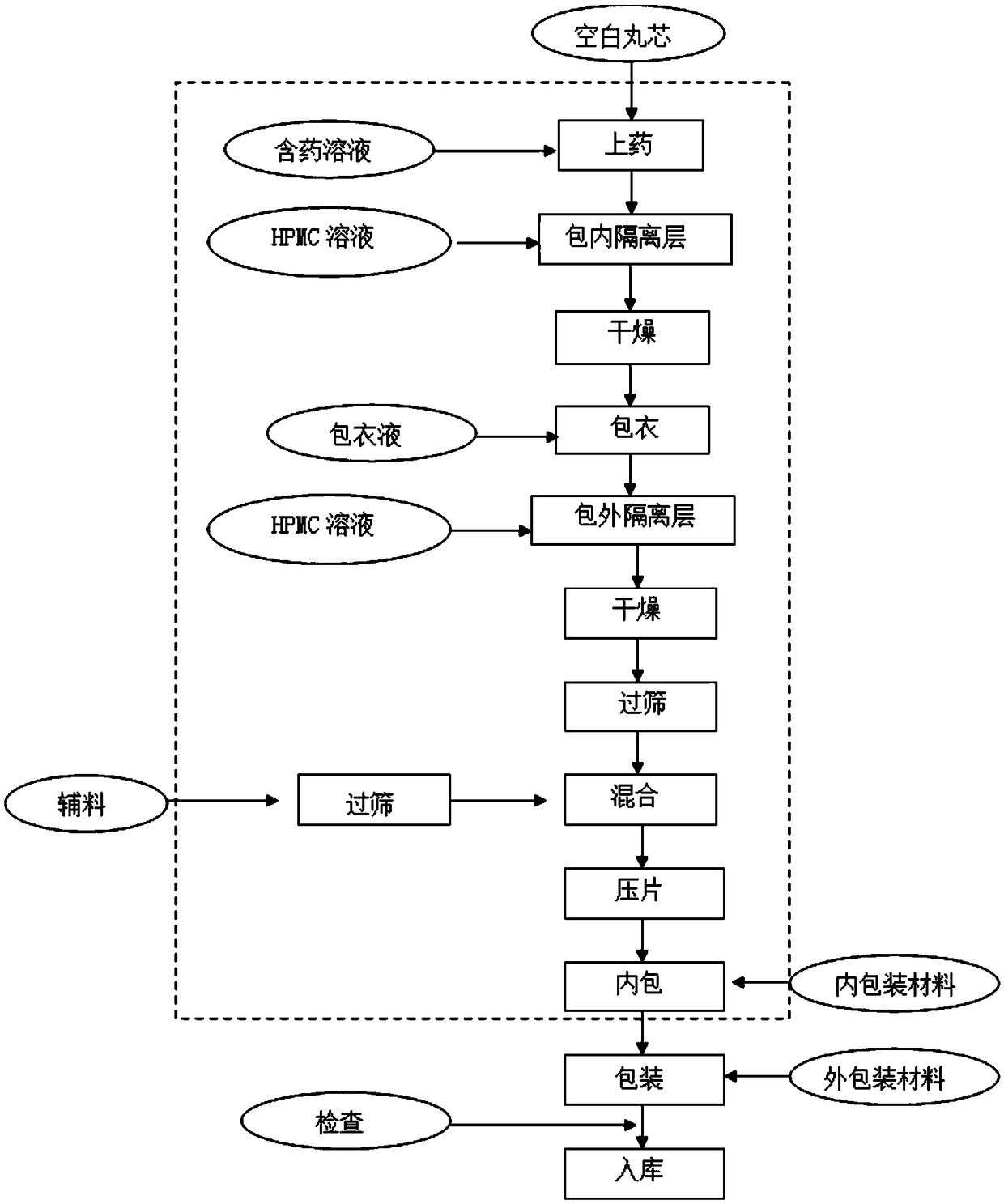
[0048] see figure 1 The preparation flowchart, the preparation method of benazepril hydrochloride chewable tablet for pets, comprises the following steps: ...
Embodiment 2
[0082] A pet benazepril hydrochloride chewable tablet, comprising the following raw materials in weight percentage: benazepril hydrochloride 5.715%, binder 4.57%, diluent 30.058%, disintegrant 1.714%, glidant 2.514%, The flavoring agent is 9.142%, and the coating taste-masking material is 46.287%.
[0083] The prescription that embodiment 2 adopts is the following table:
[0084]
[0085] In this prescription, the disintegrating agent is crospovidone.
[0086] The adhesive is hypromellose.
[0087] The flavoring agent is selected from yeast extract and beef powder seasoning.
[0088] Glidant selects talcum powder for use.
[0089] The diluent is microcrystalline cellulose.
[0090] Coating taste-masking materials include blank core pellets (coating matrix), Eudragit E100 and talcum powder (coating solution).
[0091] see figure 1 The preparation flowchart, the preparation method of benazepril hydrochloride chewable tablet for pets, comprises the following steps:
[0...
Embodiment 3
[0124] A kind of benazepril hydrochloride medicine tablet for pet, comprises following raw material in percentage by weight: benazepril hydrochloride 2%, hypromellose 2%, microcrystalline cellulose 50%, crospovidone 1.5% , talcum powder 6.5%, yeast extract and beef powder seasoning 16%, coating taste-masking material 22% (including blank core balls, Eudragit E100 and talcum powder). Among them, the ratio of blank core pellets, Eudragit E100 and talcum powder is about 64:8:9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com