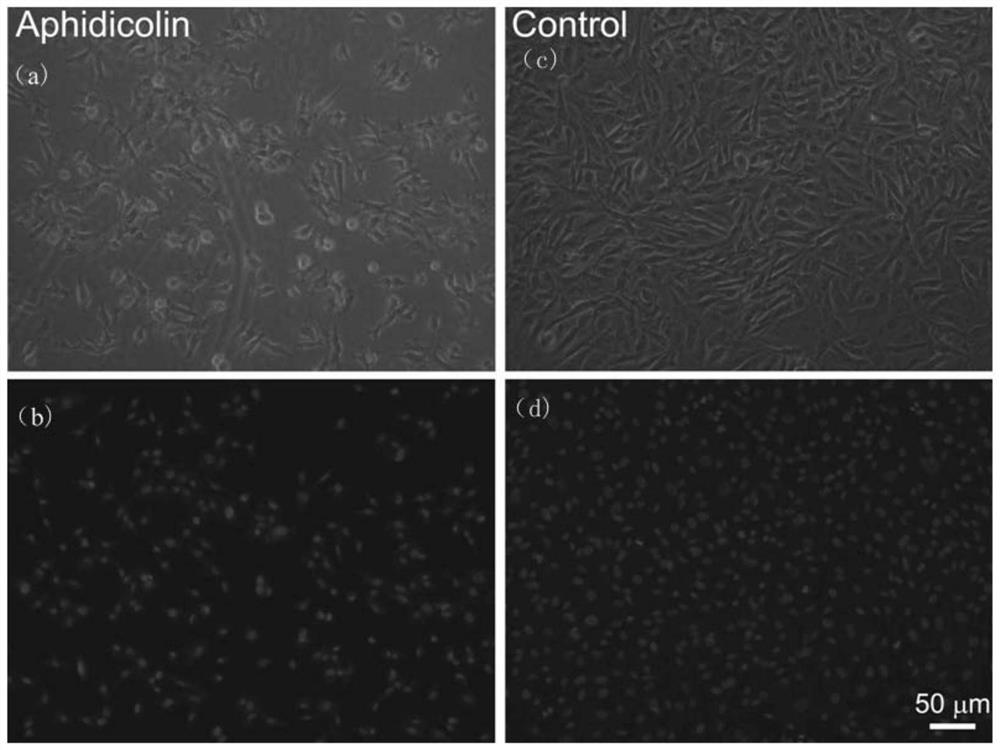
Application of interventional medical devices and aphidicolin
A technology of aphidicolin and medical equipment, which is applied in the field of interventional medical equipment and aphidicolin, and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]Aphidicolin was dissolved in DMSO solution to prepare a solution of aphidicolin with a concentration of 100 mg / mL. 100 mg of PLGA was dissolved in 10 mL of n-propyl acetate solvent to prepare a PLGA solution (drug sustained-release carrier solution) with a concentration of 10 mg / mL. Take 100 μL of aphidicolin solution and add it into 10 mL of PLGA solution to prepare a final solution with aphidicolin concentration of 1 mg / mL. The final solution was sprayed onto the surface of the L605 stent by ultrasonic spraying, and the content of aphidicolin per unit area on the surface of the stent was 10 μg / mm 2 .
Embodiment 2
[0067] Dissolve aphidicolin in DMSO solution to prepare a solution of aphidicolin with a concentration of 1 mg / mL. The aphidicolin solution was sprayed onto the surface of the stent by ultrasonic spraying. The content of aphidicolin per unit area on the surface of the stent is 10 μg / mm 2 .
Embodiment 3
[0069] Aphidicolin and PLA were simultaneously dissolved in DMSO solution to prepare a final solution with aphidicolin concentration of 10 μg / mL and PLA concentration of 10 mg / mL. Spray the final solution onto the surface of the stent by ultrasonic spraying. The content of aphidicolin per unit area on the surface of the stent is 113ng / mm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com