Bispecific chimeric antigen receptor combining two single chain antibodies and expression vector
A chimeric antigen receptor and single-chain antibody technology, applied in the field of biomedicine, can solve the problems of low effective rate of ALL, small number of clinical studies, and low effective rate, so as to reduce high recurrence rate, increase long-term survival rate, increase Effect on Complete Response Rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
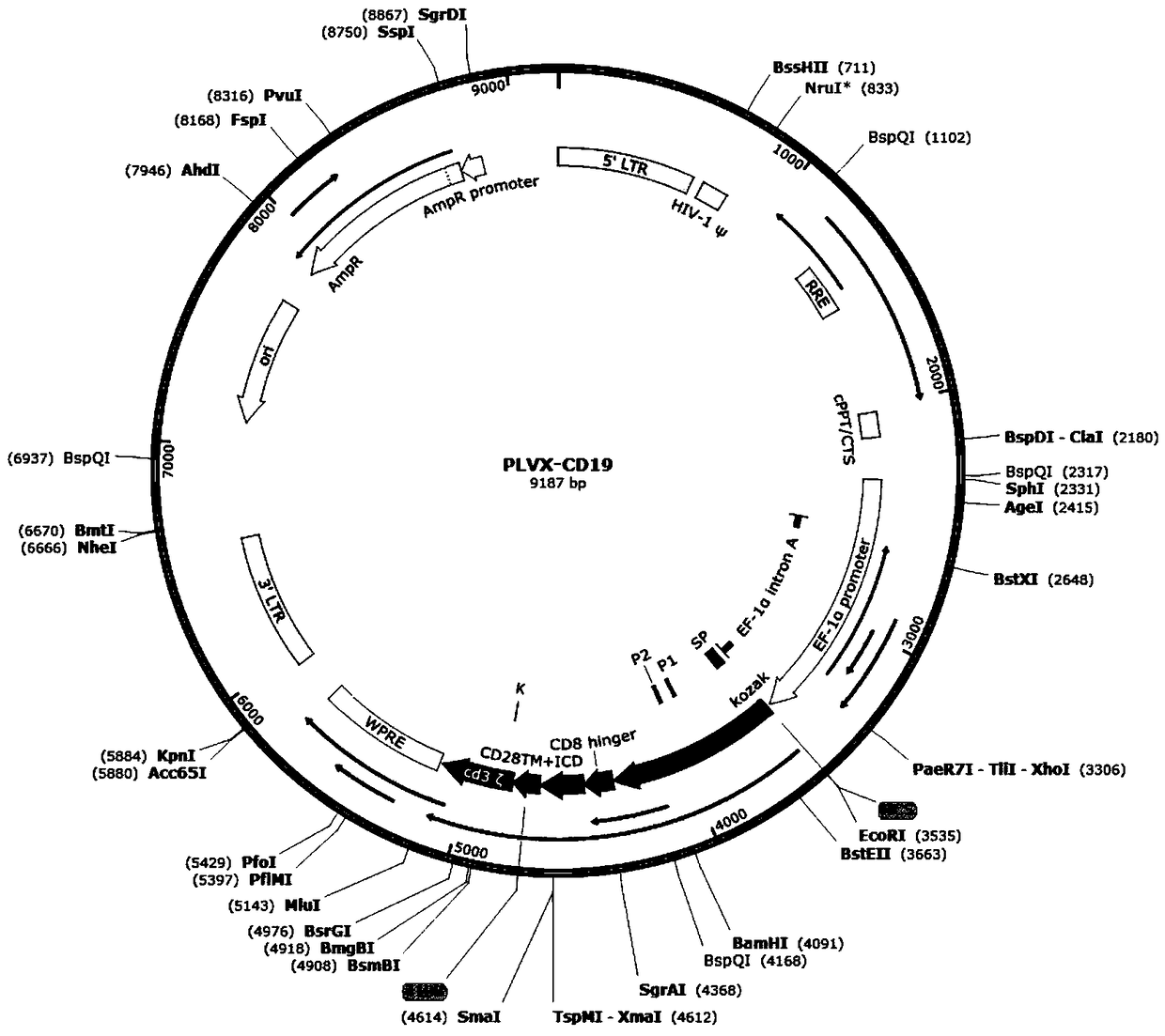
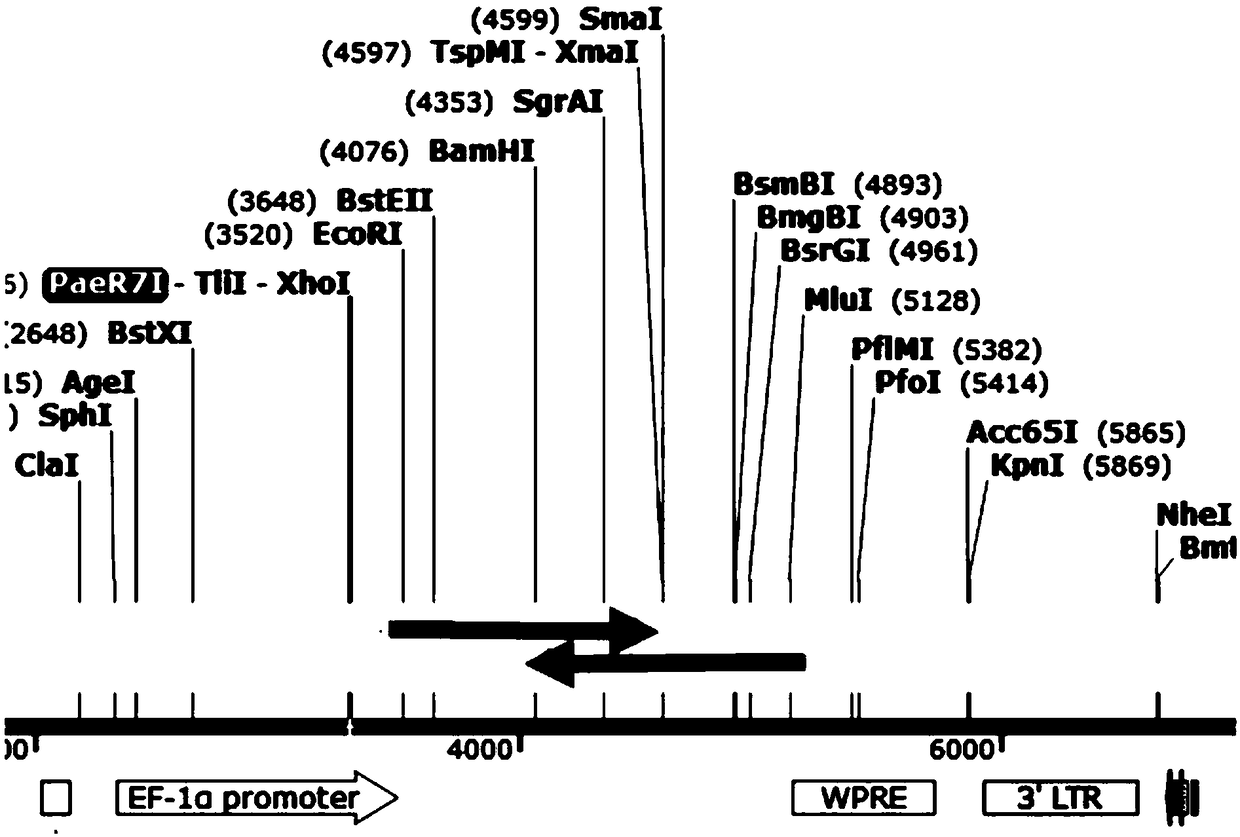
[0075] Implementation example 1, construction of recombinant lentiviral vector pLVX-CD19-CD22
[0076] The nucleotide sequence of the light chain+heavy chain of the first single-chain antibody can be called the CAR19 (CD19scFv) sequence, and the nucleotide sequence of the light chain+heavy chain of the second single-chain antibody can be called the CAR22 (CD22scFv) sequence, The sequence of CAR19 (CD19scFv) can be shown in SEQ ID NO.1, and the sequence of CAR22 (CD22scFv) can be shown in SEQ ID NO.2.
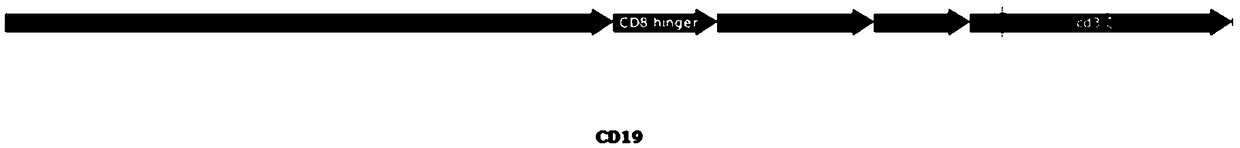
[0077] In order to further improve the design of CAR, CD28 and 4-1BB, the transmembrane region of leukocyte antigen differentiation group molecules of the third generation CAR, were used as transmembrane co-stimulatory signal molecules. The CAR sequence is as follows:
[0078] SEQ ID NO.3 is CAR19 (CAR19+CD8hinge+CD28+4-1BB+CD3ζ).
[0079] SEQ ID NO.3 is CAR22 (CAR22+CD8hinge+CD28+4-1BB+CD3ζ).
[0080] By sequence 1 (CAR19, see Figure 13 , marked as CAR19, SEQ ID NO.3) and ...
Embodiment 2
[0091] Implementation example 2, the preparation of pLVX-CD19-CD22 plasmid
[0092] Inoculate the DH5alpha strain containing the pLVX-CD19-CD22 plasmid into 250 mL of LB culture solution containing 100 μg / mL ampicillin, and culture overnight at 37° C. and 220 rpm. The culture solution was centrifuged at 6000g for 20min at 4°C, and the supernatant was discarded.
[0093] Take out Buffers P1 from the EndoFree plasma mega kit (Qiagen), add 120mL pre-cooled Buffers P1 to the centrifuged E. coli pellet, cover the cap of the centrifuge bottle, shake the centrifuge bottle vigorously to completely disperse the E. coli pellet in Buffers P1 .
[0094] Add 120mL Buffers P2 to the centrifuge bottle, put the cap on the roller mixer, slowly increase the speed to 50rpm, mix thoroughly and place at room temperature for 5min.
[0095] Add 120mL Buffers P3 to the centrifuge bottle, put the cap on the roller mixer, slowly increase the speed to the maximum speed of the roller mixer 70rpm, and m...
Embodiment 3
[0117] Implementation example 3, preparation of recombinant lentivirus LV Anti-CD19-CD22 CAR
[0118] Insert 130.0~140.0×10 in multilayer cell culture bottle (Hyperflask) (Corning) 6 Number of 293T cells (Takara), a total of 560 mL DMEM complete medium (50 mL fetal bovine serum, 5 mL Antibiotic-Antimycotic (100×)), at 37 °C with 5% CO 2 Incubate for 24 hours in the incubator. Add DMEM complete medium mixed with 320 μg plasmid (pLVX-CD19-CD22: gag plasmid: vsvg plasmid=6:3:2) into a 960 μg PEI tube, vortex, and equilibrate at room temperature for 10 minutes. Mix the above-mentioned 35mL PEI-plasmid mixture with 525mL DMEM complete medium, and replace it into the above-mentioned multi-layer cell culture flask. Place the multilayer cell culture flask at 37 °C with 5% CO 2 After 3 days in the incubator, the cell culture supernatant was collected.
[0119] After the supernatant was centrifuged at 4000 rpm (or 3000 g) for 30 min, cryonase (Takara) was added to the centrifuged su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com