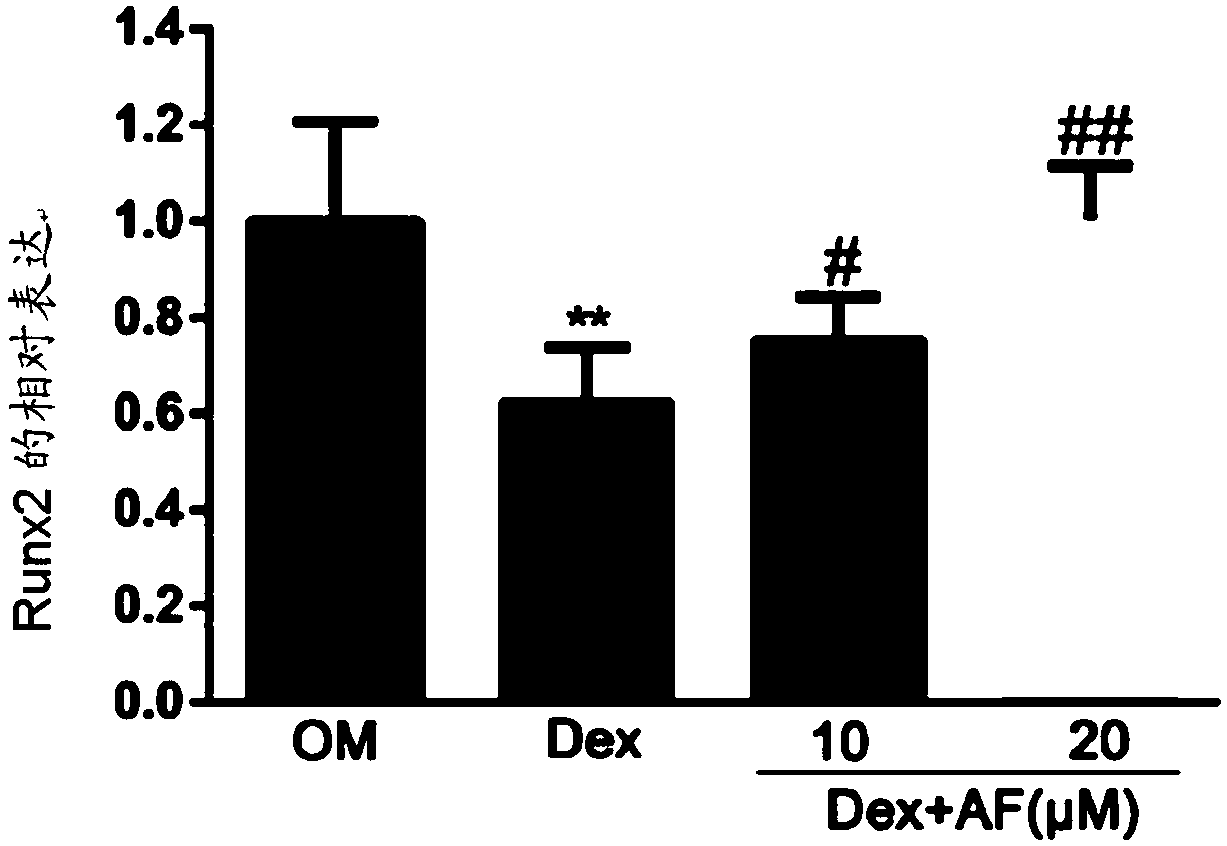Method for relieving glucocorticoid side effects
A technology for glucocorticoids and side effects, which is applied in the field of alleviating the side effects of glucocorticoids, and can solve problems such as increased upper gastrointestinal bleeding or perforation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 The effect of the compound of formula I of the present invention on osteogenesis-related genes in the inhibition of MC3T3-E1 cell osteogenic differentiation by dexamethasone
[0039] MC3T3-E1 cells were cultured in groups
[0040] (1) After the cells were cultured to a confluence of 80%, the cells were digested, and the digested cells were prepared into a uniform single-cell suspension with α-MEM complete medium, and the cell density was adjusted to 10 5 cells / well; take a 6-well plate, inoculate 2ml of cell suspension in each well, and culture in a carbon dioxide incubator.
[0041] (2) Grouping treatment: 24 hours after seeding the plate, the cells grew to reach a confluence of 80% to 90%, and the cells were divided into groups, 3 wells / group, and the corresponding medium was replaced every other day, 2ml / well. Grouped as follows:
[0042] ① OM induction medium α-MEM culture treatment group (OM induction medium composition: 10mM β-sodium glycerophosphate, ...
Embodiment 2
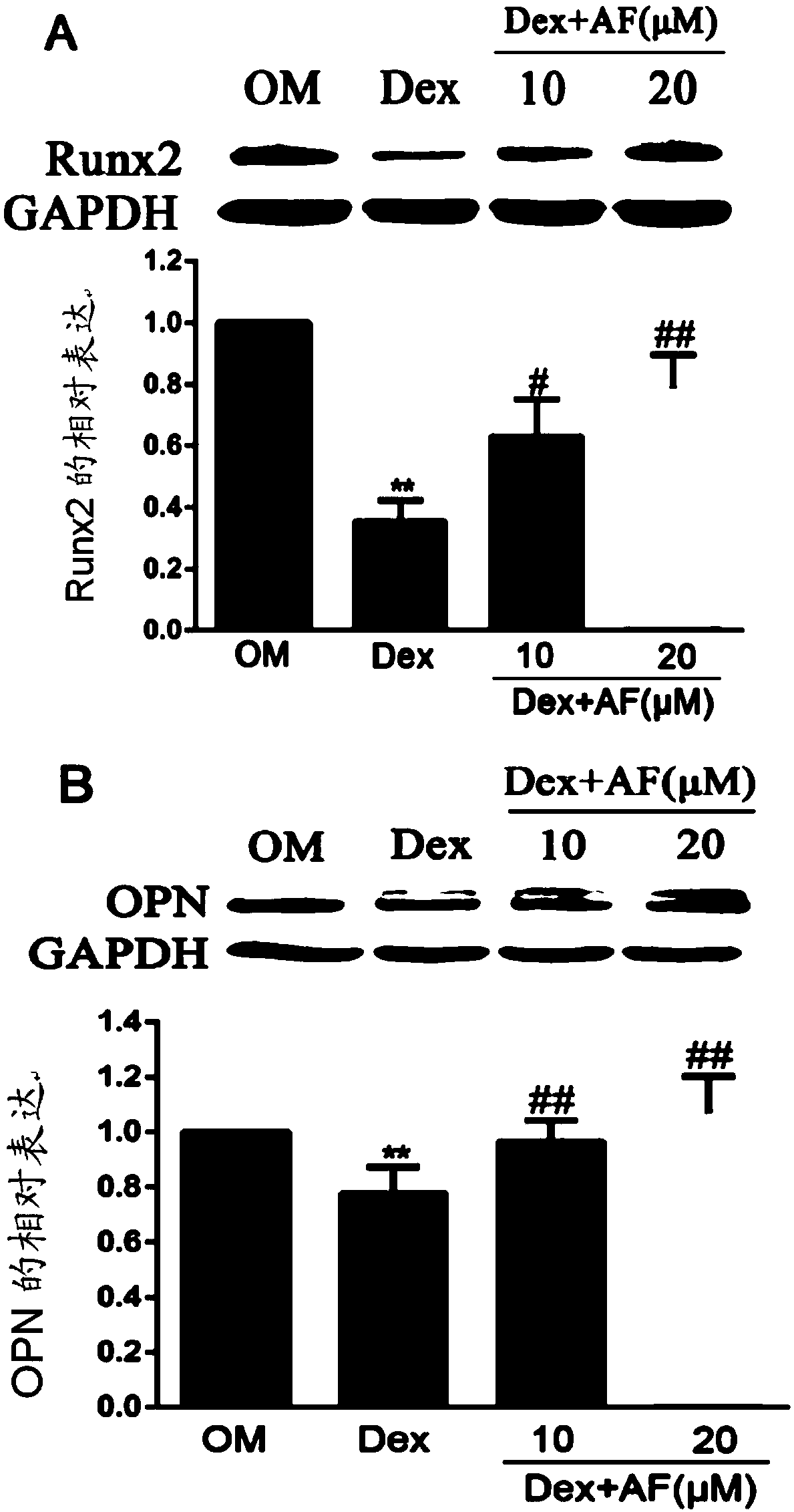
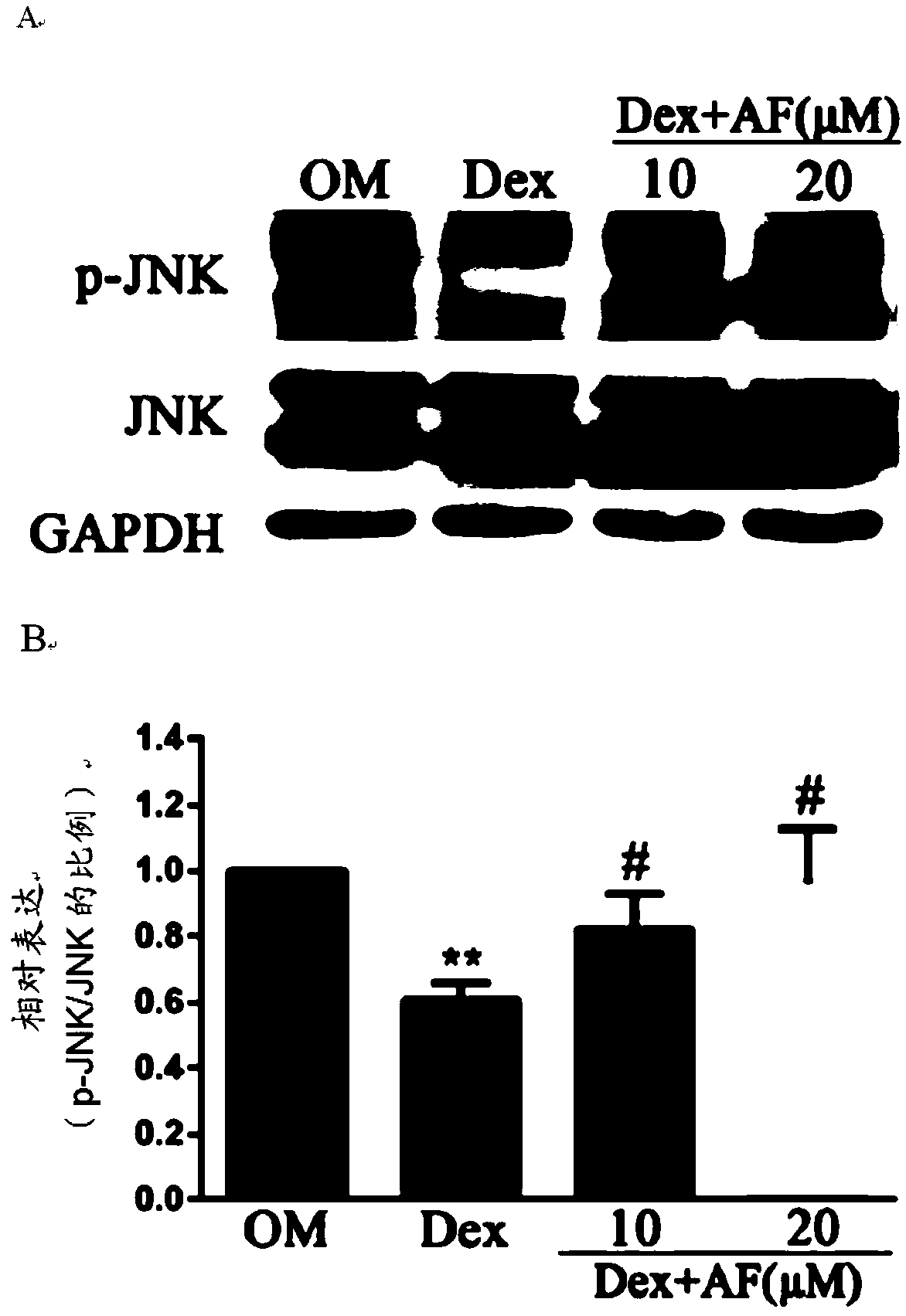
[0057] Example 2 The effect of the compound of formula I of the present invention on the expression of osteogenesis-related proteins and the JNK signaling pathway in inhibiting the osteogenic differentiation of MC3T3-E1 cells.
[0058] (1) Cell grouping: 24 hours after seeding the plate, the cell growth reached 80%-90% confluence, and the cells were grouped into 3 wells / group, and 2 ml / well of the corresponding medium was replaced every other day. Grouped as follows:
[0059] ① OM induction medium α-MEM culture treatment group
[0060] ②10 -6 M Dex+OM induction medium α-MEM culture treatment group
[0061] ③10μM compound of formula I of the present invention+10 -6 M Dex+OM induction medium α-MEM culture treatment group
[0062] ④ 20μM compound of formula I of the present invention+10 -6 M Dex+OM induction medium α-MEM culture treatment group
[0063] (2) Cell total protein was extracted on the 5th day of cell group culture.
[0064] (3) Determination of protein concentr...
Embodiment 3
[0069] Example 3 The protective effect of the compound of formula I of the present invention on the reduction of mineralization formation of MC3T3-E1 cells inhibited by dexamethasone.
[0070] (1) Cell inoculation and culture: the digested cells were prepared into a uniform single-cell suspension with α-MEM complete medium, and the cell density was adjusted to 2.5×10 4 cells / well; take a 24-well plate, inoculate 500 μl of cell suspension in each well, and culture in a carbon dioxide incubator.
[0071] (2) Cell grouping treatment: 24 hours after cell seeding, the cells grew to reach 80%-90% confluence, and the cells were grouped into 4 wells / group, and the corresponding medium was replaced every other day, 500 μl / well. Grouped as follows:
[0072] ① OM induction medium α-MEM culture treatment group
[0073] ②10 -6 M Dex+OM induction medium α-MEM culture treatment group
[0074] ③10μM compound of formula I of the invention+10 -6 M Dex+OM induction medium α-MEM culture trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com