Infusion preparation for dialysis patient
A technology for patients and pharmaceutical preparations, applied in blood diseases, drug delivery, extracellular fluid diseases, etc., to achieve the effect of reducing the dosage and improving the effect of dialysis treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: Preparation of anti-anemia preparation (amino acid infusion)
[0123] The ingredients shown in Table 1 below were dissolved in water for injection in the amounts shown in the table. The solution was sterile filtered and placed in a glass bottle. The bottle was sealed and autoclaved to prepare an anti-anemia preparation (amino acid infusion).
[0124] The pH of the preparation is 6.6-7.6, and the osmotic pressure ratio is about 2.
[0125] Table 1:
[0126] ingredient 200mL L-Isoleucine 1.500g L-Leucine 2.000g L-lysine (as acetate) 1.400g L-methionine 1.000g L-phenylalanine 1.000g L-threonine 0.500g L-tryptophan 0.500g L-valine 1.500g
[0127] L-Alanine 0.600g L-Arginine 0.600g L-Aspartic Acid 0.050g L-glutamic acid 0.050g L-histidine 0.500g L-Proline 0.400g L-serine 0.200g L-tyrosine 0.100g Glycine 0.300g Total amino acids 12.200g A...
Embodiment 2
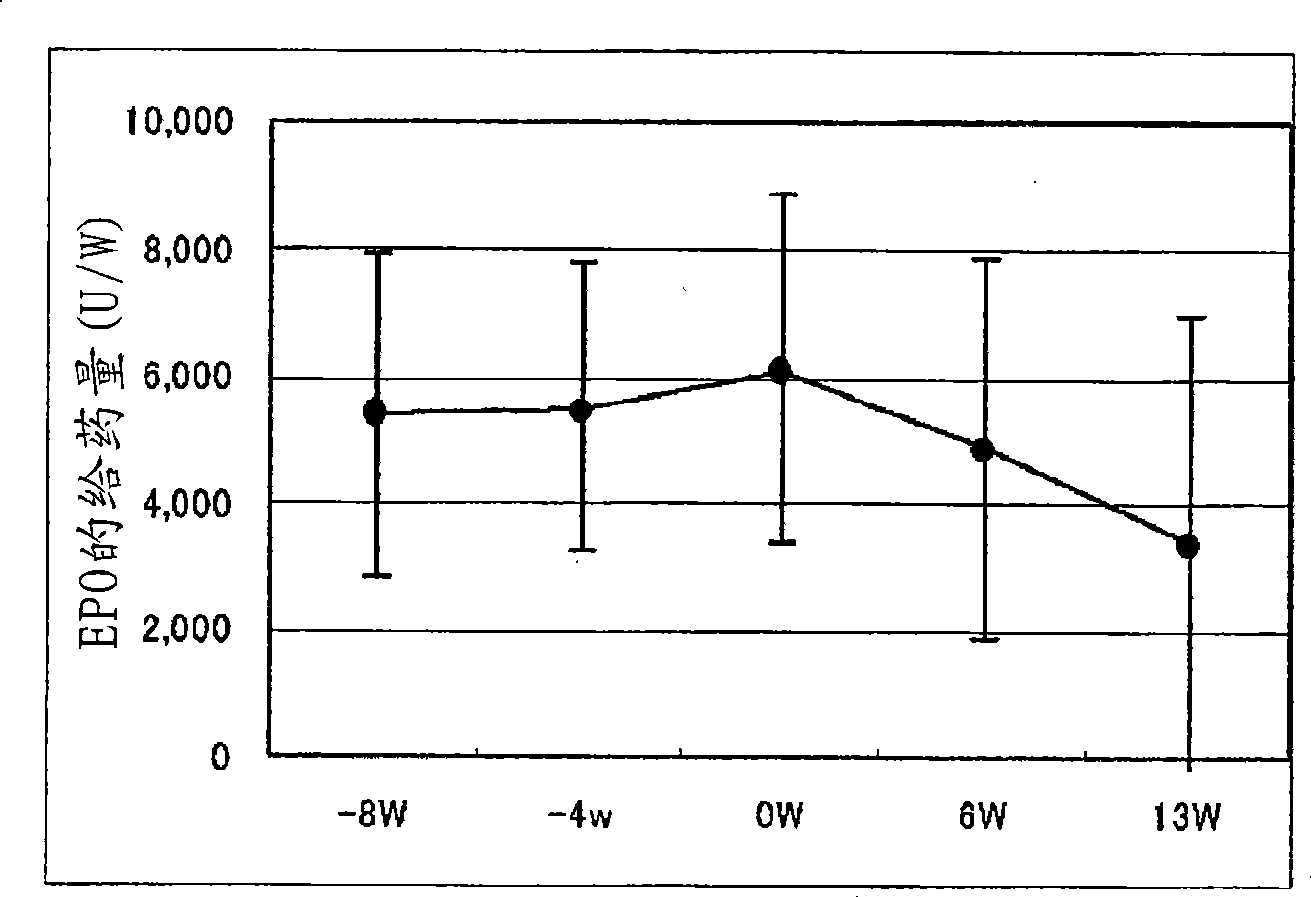
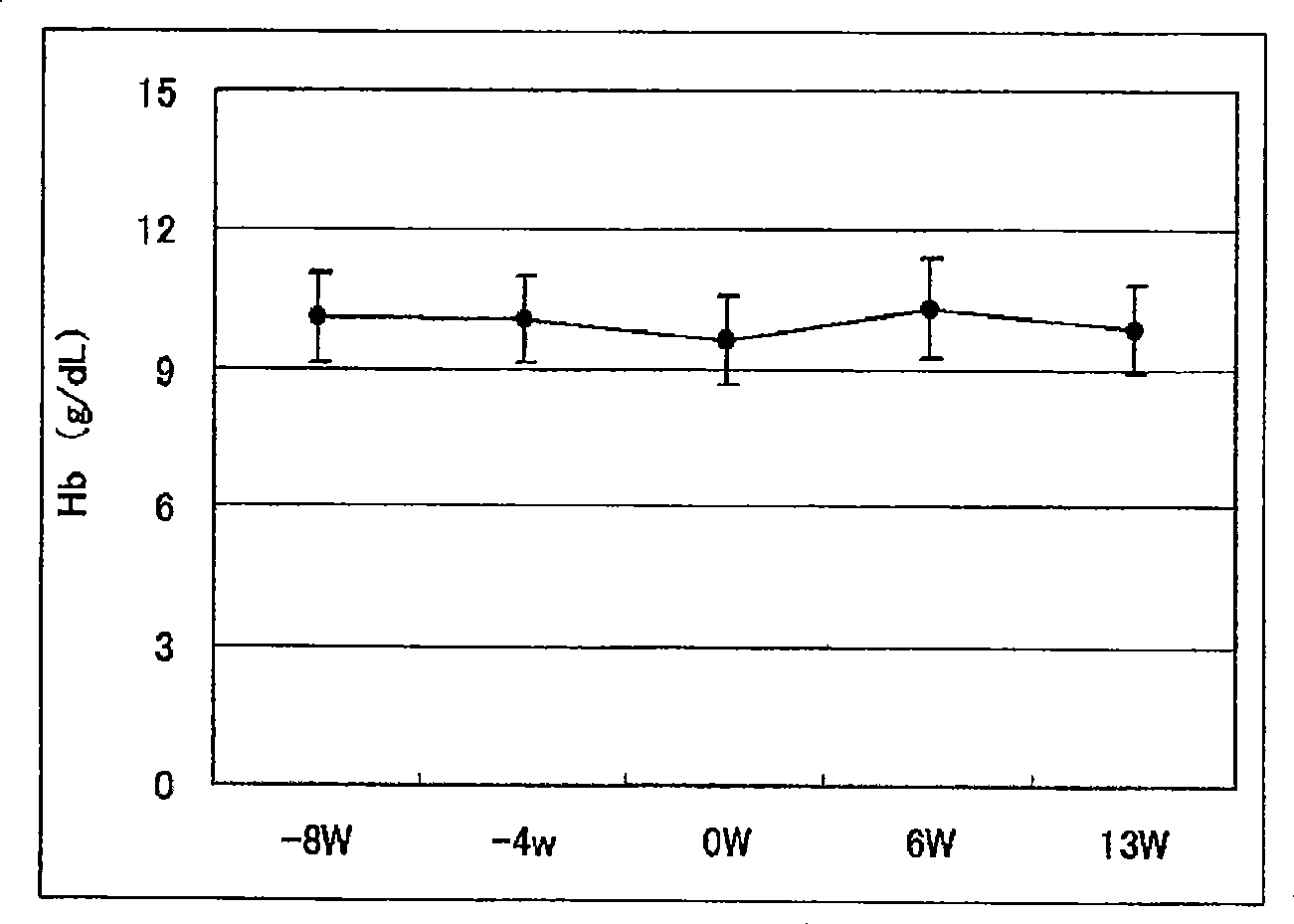
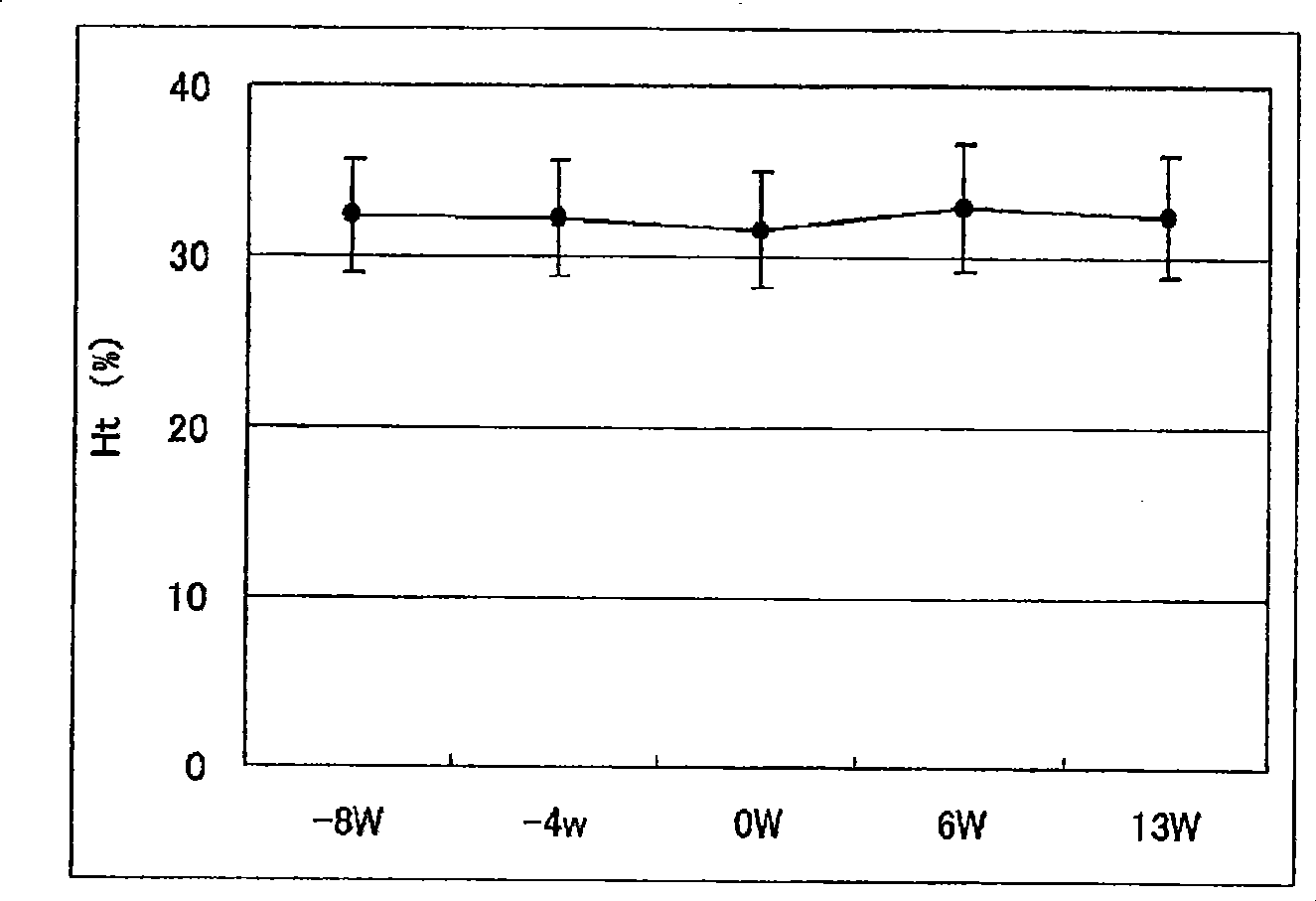
[0129] Example 2. Determination of the ability of pharmaceutical preparations to improve anemia and reduce the dosage of erythropoietin
[0130] 18 cases of chronic renal failure patients receiving hemodialysis (HD: 9 cases) or hemodiafiltration (HFD: 9 cases) were administered the exemplary anti-anemia preparation of the present invention to check that the preparation improves anemia and reduces the administration of erythropoietin Capacity.
[0131] [method]
[0132] The commercially available amino acid infusion solution "NEOAMYU (registered trademark)" (200 mL formulation, Ajinomoto Pharma Co., Ltd.) prepared according to Example 1 was used.
[0133]In the week before the administration period, necessary diagnosis, inspection and testing were performed on the first dialysis day (week 1 or week 2). From 1 week after diagnosis to 12 weeks and 1 day (the 37th dialysis), the "NEOAMYU" formulation was continuously administered. Specifically, 1 bottle (200 mL) of the preparation was...
Embodiment 3
[0140] Example 3: Determination of the ability of the above pharmaceutical preparations to control serum phosphorus levels
[0141] The exemplary serum phosphorus control preparation of the present invention was administered to 8 patients with chronic renal failure undergoing hemodialysis (HD) to check changes in serum phosphorus levels.
[0142] [method]
[0143] A commercially available amino acid infusion solution "NEOAMYU" (200 mL formulation, Ajinomoto Pharma Co., Ltd.) prepared according to Example 1 was used.
[0144] In the week before the administration period, necessary diagnosis, inspection and testing were performed on the first dialysis day (week 1 or week 2). From 1 week after diagnosis to 12 weeks and 1 day (the 37th dialysis), the "NEOAMYU" formulation was continuously administered. Specifically, 1 bottle (200 mL) of the preparation was injected into the venous side of the dialysis circuit during each dialysis. The preparation was input throughout the course of dia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com