Preparation method for complex containing o-benzhydryl substituted alpha-diimine nickel (II) used for fluorination of ethylene polymerization
A technology of benzhydryl and nickel diimide, which is applied in the field of olefin polymerization, can solve the problems of limited research and achieve the effect of large steric hindrance, promotion of chain growth rate, and reduction of transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Synthesis of 2-benzhydryl-4-bromo-6-fluoroaniline:
[0044]
[0045] In a 50ml round bottom flask, add 2-fluoro-4-bromoaniline (1.89g, 10mmol) and diphenylmethanol (1.84g, 10mmol), stir the mixture and slowly heat up to 120°C, add anhydrous chlorine to the mixture Zinc chloride (0.68g, 5mmol), concentrated hydrochloric acid (37%, 0.37g, 10mmol), heated to 160°C, reacted for 1 hour (exothermic and strong bubbling), after cooling to room temperature, the solid was dissolved in 50mL CH 2 Cl 2 , washed three times with saturated NaOH aqueous solution, and the organic layer was washed with anhydrous MgSO 4 Dry, concentrate under reduced pressure, add ethanol to precipitate a white solid, filter and dry to obtain 2.91 g of 2-benzhydryl-4-bromo-6-fluoroaniline with a yield of 82%.
[0046] Its reaction formula is as follows:
[0047]
[0048] (2) Ligand (2-CHPh 2 -4-Br-6-FPh) 2 Synthesis of DABAn:
[0049]
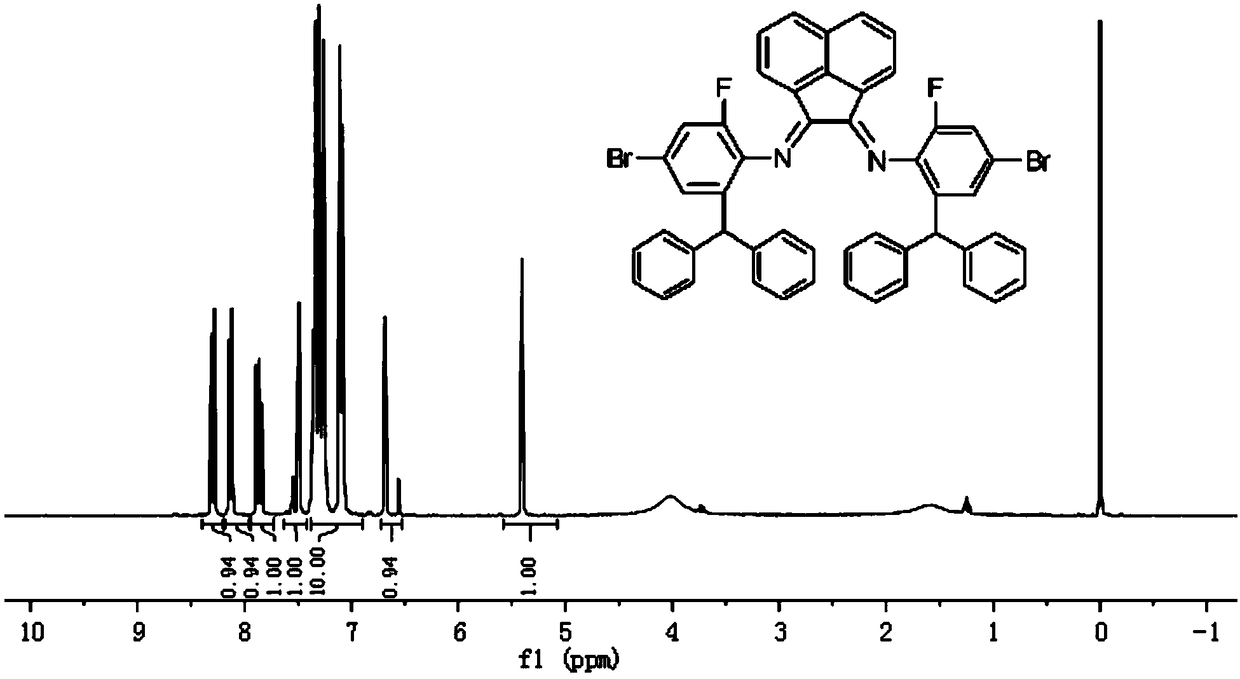
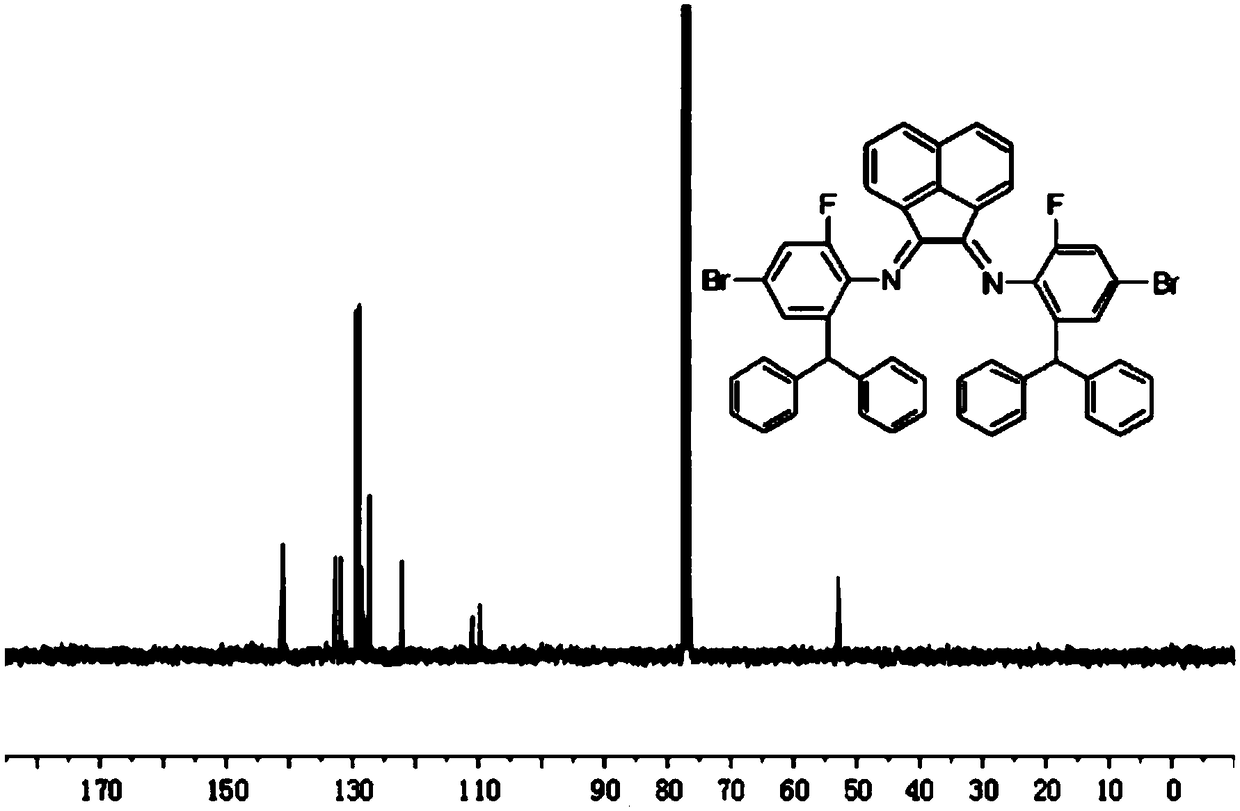
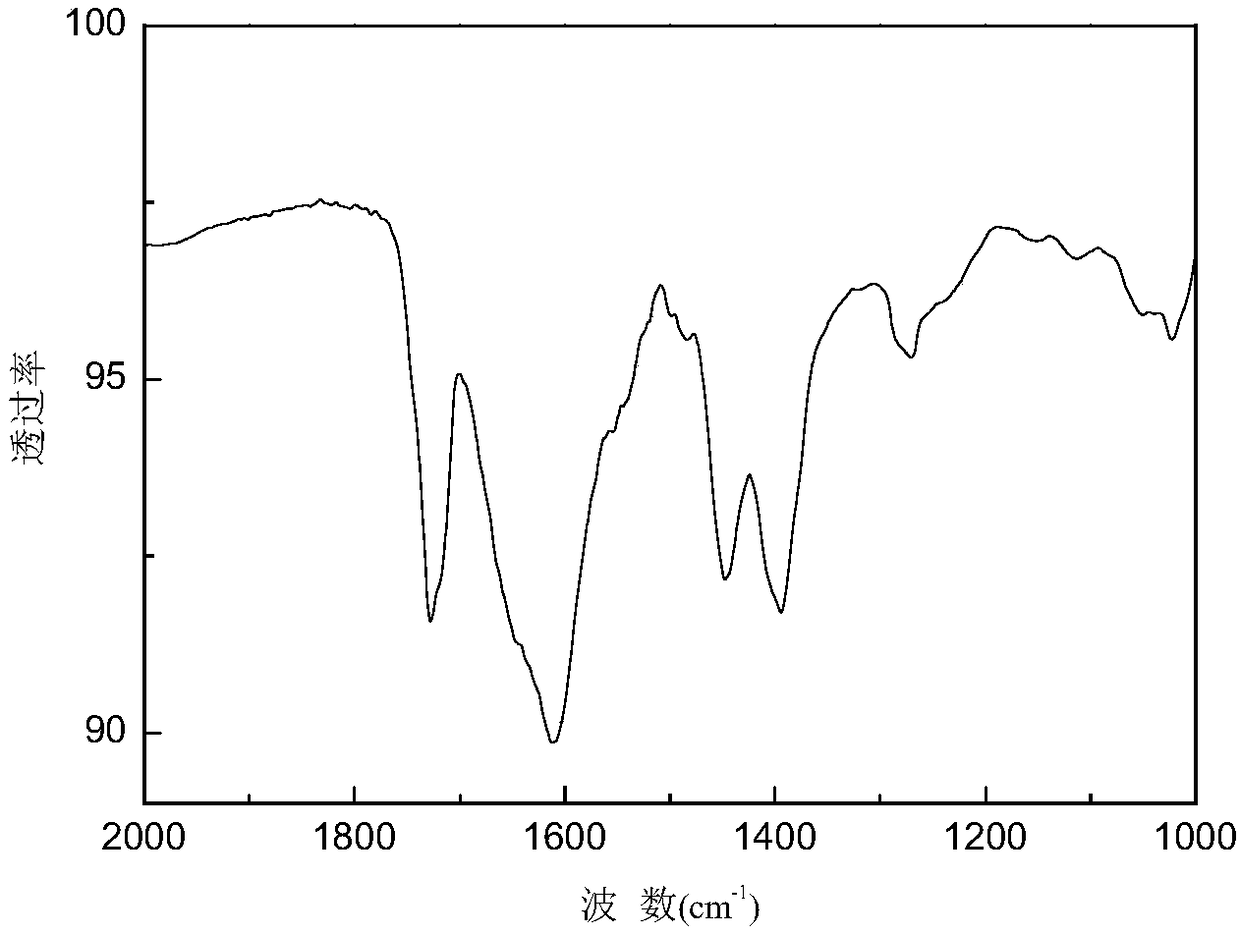
[0050] Dissolve 2-benzhydryl-4-bromo-6-fluoroanili...
Embodiment 2
[0060] (1) Synthesis of 2-benzhydryl-4-chloro-6-fluoroaniline:
[0061]
[0062] In a 50ml round bottom flask, add 2-fluoro-4-chloroaniline (1.45g, 10mmol) and diphenylmethanol (1.84g, 10mmol), stir the mixture and slowly heat up to 120°C, add anhydrous chlorine to the mixture Zinc chloride (0.68g, 5mmol), concentrated hydrochloric acid (37%, 0.37g, 10mmol), heated to 160°C, reacted for 1 hour (exothermic and strong bubbling), after cooling to room temperature, the solid was dissolved in 50mL CH 2 Cl 2 , washed three times with saturated NaOH aqueous solution, and the organic layer was washed with anhydrous MgSO 4 Dry, concentrate under reduced pressure, add ethanol to precipitate a white solid, filter and dry to obtain 2.61 g of 2-benzhydryl-4-chloro-6-fluoroaniline with a yield of 84%.
[0063] Its reaction formula is as follows:
[0064]
[0065] (2) Ligand (2-CHPh 2 -4-Cl-6-FPh) 2 Synthesis of DABAn:
[0066]
[0067] Dissolve 2-benzhydryl-4-chloro-6-fluoroa...
Embodiment 3
[0077] (1) Synthesis of 2-benzhydryl-4,6-difluoroaniline:
[0078]
[0079] In a 50ml round bottom flask, add 2,4-difluoroaniline (1.29g, 10mmol) and diphenylmethanol (1.84g, 10mmol), stir the mixture and slowly raise the temperature to 120°C, add anhydrous chloride Zinc (0.68g, 5mmol), concentrated hydrochloric acid (37%, 0.37g, 10mmol), heated to 160°C, reacted for 1 hour (exothermic and strong bubbling), after cooling to room temperature, the solid was dissolved in 50mL CH 2 Cl 2 , washed three times with saturated NaOH aqueous solution, and the organic layer was washed with anhydrous MgSO 4 Dry, concentrate under reduced pressure, add ethanol to precipitate a white solid, filter and dry to obtain 2.36 g of 2-benzhydryl-4,6-difluoroaniline, and the yield is 80%.
[0080] Its reaction formula is as follows:
[0081]
[0082] (2) Ligand (2-CHPh 2 -4,6-diFPh) 2 Synthesis of DABAn:
[0083]
[0084] Dissolve 2-benzhydryl-4,6-difluoroaniline (1.24g, 4.2mmol) and a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com