Interlocking network crosslinking polymer based on reversible covalent bond as well as preparation method and application thereof
A cross-linked polymer and network cross-linking technology, which is applied in the field of interlocking network cross-linked polymers and its preparation, can solve the problems of reducing or not being able to maximize the "forced compatibility" effect and mechanical properties of network interpenetration , to achieve the effect of simple and easy preparation method, easy composition and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 intermediate containing reversible DA bond cross-linked network D: ED900-DA
[0089] This example provides a cross-linked network D: ED900-DA containing reversible DA bonds. The synthesis steps are as follows: 16g of glycidyl furfuryl ether and 18.81g of N,N''-(4,4''-methylenebis Phenyl)bismaleimide was dissolved in 150mL tetrahydrofuran, added to a round-bottomed three-neck flask equipped with a condensation device, a nitrogen protection device, and a mechanical stirring device, reacted at 70°C for 24h, cooled to room temperature, and distilled under reduced pressure After removing the solvent, a yellow viscous liquid was obtained. Adopt silica gel chromatographic column to purify, eluent is dichloromethane / methanol=50:1, collect corresponding eluent and distill under reduced pressure and remove solvent to obtain white powdery solid product, product structural formula is shown in the following formula, and the nuclear magnetic spectrum of product sees Figure...
Embodiment 2
[0091] Example 2 The intermediate contains a dynamic reversible C-ON bond cross-linked network C: 4-CON
[0092] This example provides a cross-linked network C: 4-CON containing dynamic reversible C-ON bonds, the synthesis process is as follows: 5g 4-OH-TEMPO and 8.37g 2,2'- azo -N-(2-Hydroxyethyl)propionamide] was dissolved in 150mL of dimethylformamide, added to a round-bottomed three-neck flask equipped with a condensation device, a nitrogen protection device, and a mechanical stirring device, and reacted at 120°C for 3h After cooling to room temperature, the solvent was distilled off under reduced pressure to obtain a yellow viscous liquid. Purified by silica gel chromatography, the eluent is petroleum ether / ethyl acetate=5:3 and pure ethyl acetate in turn, the corresponding eluent is collected and evaporated under reduced pressure to remove the solvent to obtain a colorless oily liquid product, the product structure is shown in the following formula , the H NMR spectrum ...
Embodiment 3
[0094] Embodiment 3 interlocking network CD-13
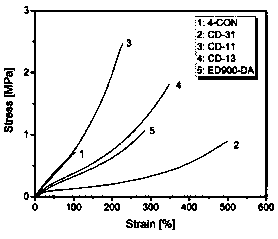
[0095] This example provides an interlocking network CD-13, which consists of 25 parts of a cross-linked network C: 4-CON containing a dynamic reversible C-ON bond (provided in Example 2) and 75 parts of a cross-linked network containing a reversible DA bond D: ED900-DA (provided in Example 1), prepared by mixing after dissociation.
[0096] Preparation of interlocking network CD-13: take 7.5g of the product ED900-DA obtained in Example 1, put it in 150ml of dimethylformamide after crushing, fully swell for 24 hours, and then add it to the In a three-necked round-bottomed flask with a mechanical stirring device, stir at 130°C for 30 minutes to obtain a transparent solution. Take 2.5 g of the product 4-CON obtained in Example 2, put it in 50 ml of dimethylformamide after crushing, fully swell for 24 hours, and then add it to a round-bottomed three-neck flask equipped with a condensation device, a nitrogen protection device, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com