Hydroformylation process
A formylation catalyst and catalyst technology, applied in organic chemistry, carbon-based compound preparation, chemical instruments and methods, etc., can solve problems such as high and heavy components, and achieve the effect of low capital cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0070] All parts and percentages in the following examples are by weight unless otherwise indicated. Pressures are given in absolute unless otherwise indicated.
example 1
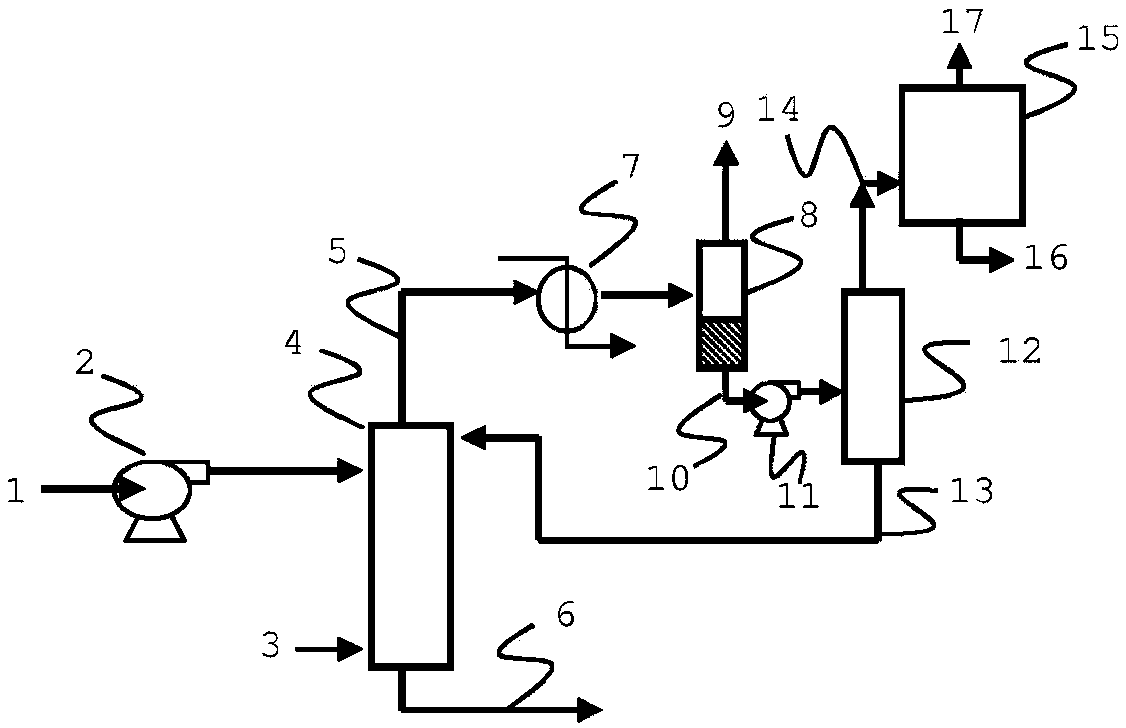
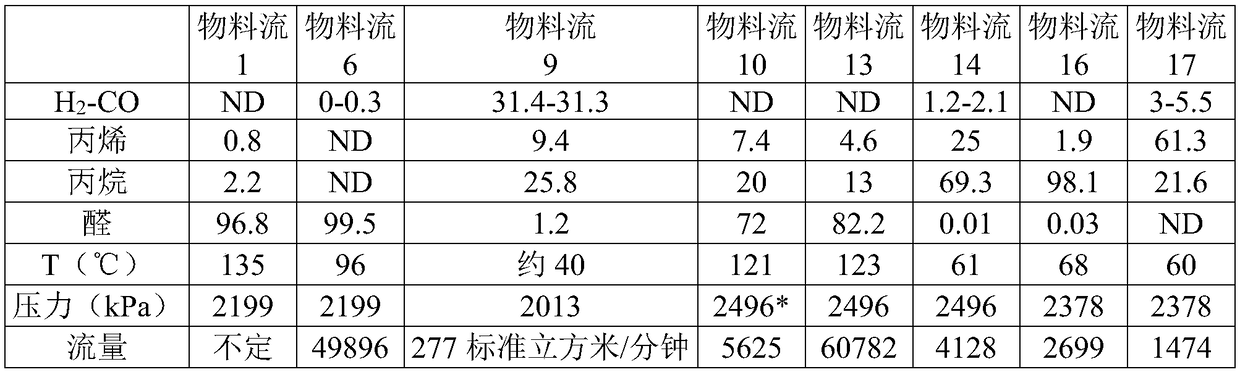
[0072] Using chemical process simulation software to model a conventional oxo reaction system with two identical CSTR reactors (which is similar to that in Figure 21D of Process Economics Program Report 21D, OXO ALCOHOLS) (December 1999) The one described in Figure 4.6 is available from IHS Inc.). No compressor was used in the simulation. The catalyst was a typical Rh-TPP catalyst as described in U.S. Patent No. 4,148,830 (Example 13), and the reaction conditions were essentially those of Example 13 of said patent for propylene, except that the first reactor The initial target rhodium concentration was 250-350 ppm Rh. The TPP concentration in the reactor was about 10-11%. Based on an olefin feed rate of 33700 kg / h propylene (90-95% purity), the selected process conditions and unrefined aldehyde production are shown in Table 1:
[0073] based on figure 1 , the selected process conditions and the unrefined aldehyde production rate are shown in Table 1 (the material flow sequ...
example 2
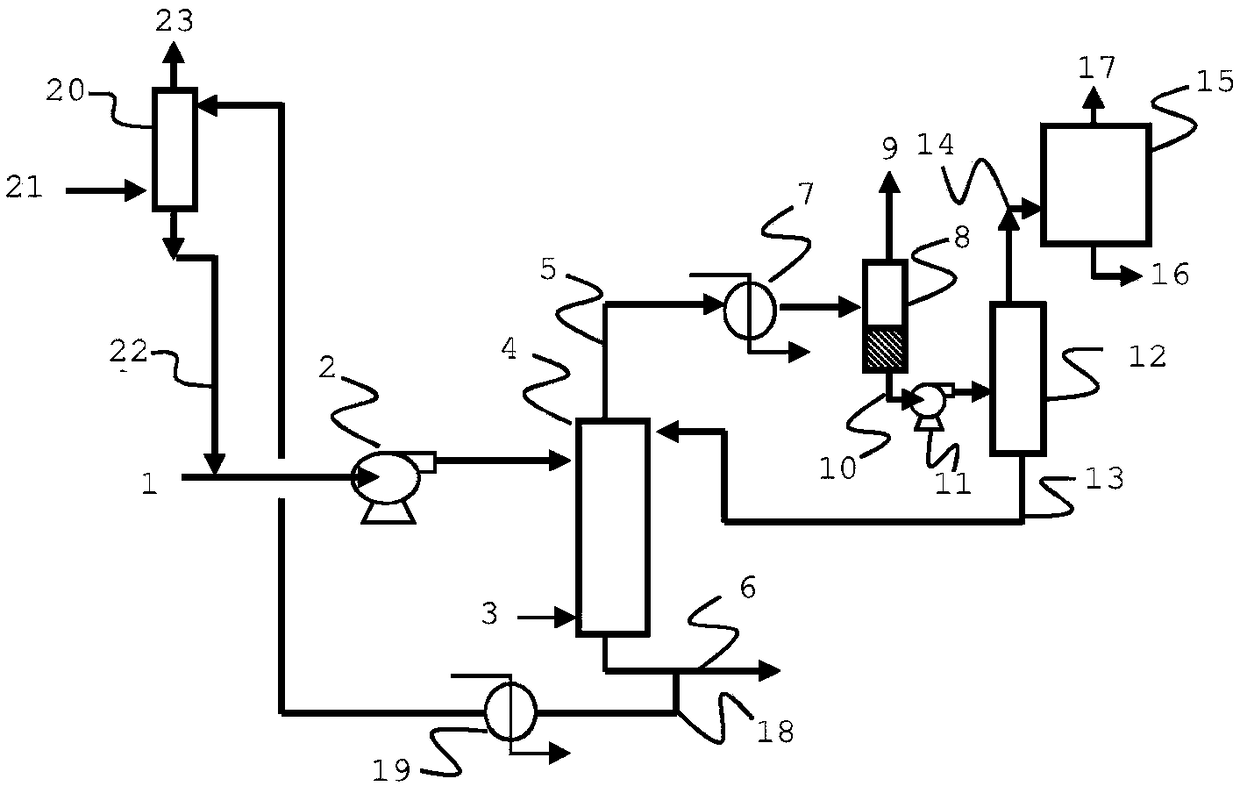
[0079] as in figure 2 Depicted is an absorption column (20) in the form of an effluent scrubber for scrubbing the purge effluent from Reactor #2 (Oxoalcohol Process Economics Project Report 21D (December 1999) in Figure 4.6 (7) equivalent). Unless otherwise stated, references to equipment or streams in this Example 2 correspond to figure 2 . The stream from the purge effluent (21) is added to the middle of the absorption column (20). The absorption column (20) used for this simulation consisted of 33 trays. The output from the high-pressure evaporator (equivalent of stream (11) in Figure 4.6 of Oxoalcohol Process Economics Project Report 21D (December 1999)) is fed (20) at the bottom of the absorption column, and the combined The gas stream is contacted with the stripped aldehyde and the bottoms stream is combined with stream (10). The results shown in Table 2 show very high recovery of olefins from the effluent with very little loss of aldehydes. The remainder of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com