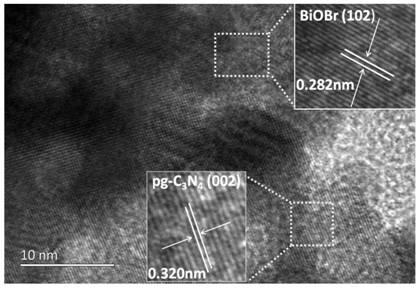
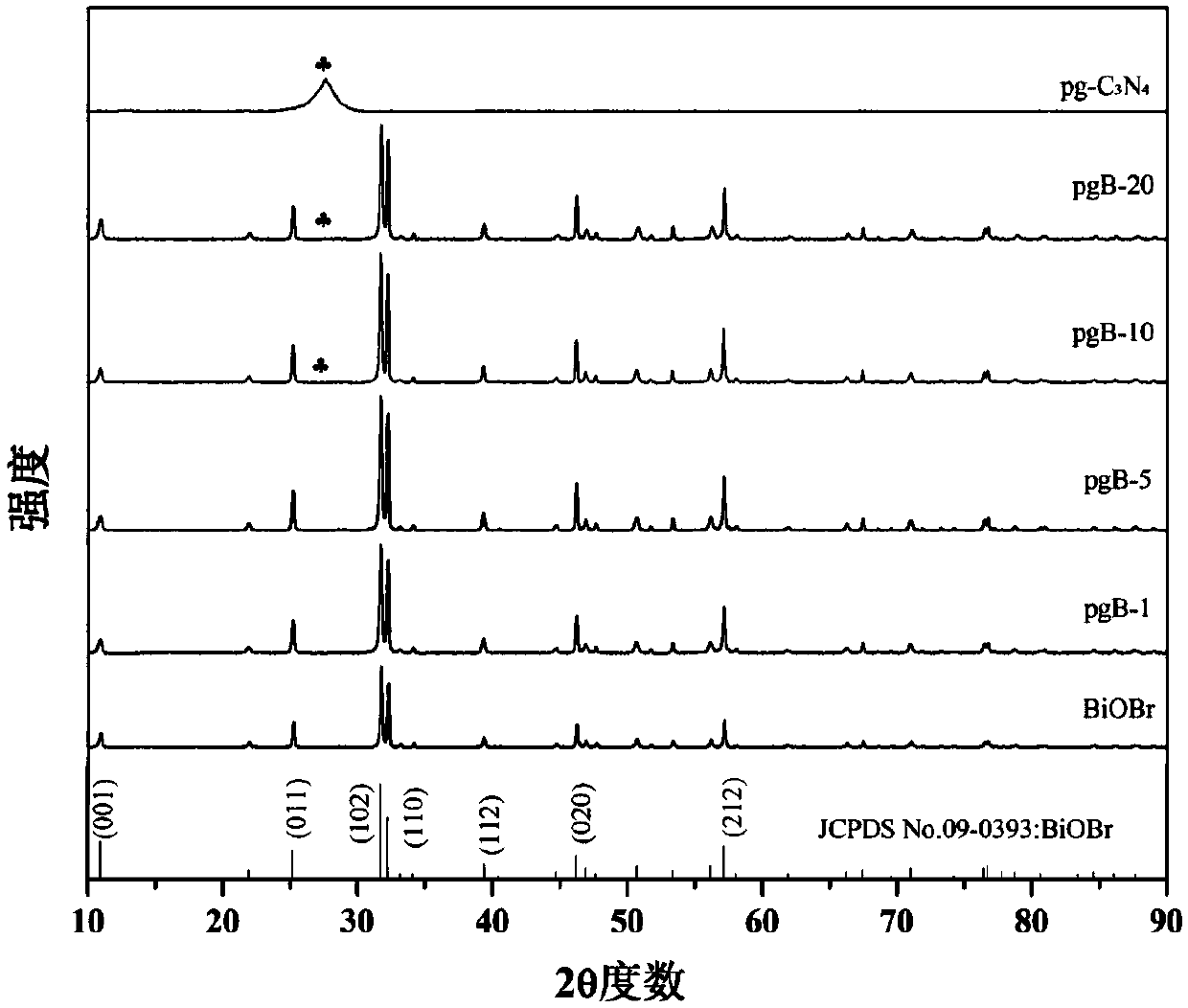
Protonated g-C3N4/BiOBr heterojunction photocatalyst and preparation method
A photocatalyst, g-c3n4 technology, applied in the field of photocatalytic materials, achieves the effects of high removal efficiency, good application prospects and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the present invention comprises the following steps:
[0036] 1) Protonated g-C 3 N 4 Preparation: add g-C 3 N 4 The precursor is moved into a semi-closed alumina crucible, placed in a muffle furnace and calcined by air to obtain g-C 3 N4 Photocatalyst particles. Weigh g-C 3 N 4 Photocatalyst particles are dispersed in hydrochloric acid solution, stirred at room temperature for 3-5 hours, washed with deionized water until neutral, and the light yellow g-C 3 N 4 The photocatalyst particles were dried in an oven to obtain protonated g-C 3 N 4 catalyst of light.
[0037] 2) Curd-like protonated g-C in response to visible light 3 N 4 / BiOBr heterojunction photocatalyst preparation: Weighing protonated g-C 3 N 4 Dissolve photocatalyst and bismuth nitrate in 100mL deionized water, add 9mL acetic acid solution during stirring, after stirring at room temperature, add potassium bromide and 3mmol sodium acetate to the solution, continue sti...
Embodiment 1
[0050] Step 1: Put g-C 3 N 4 The precursor (melamine) is moved into a semi-closed alumina crucible, placed in a muffle furnace and calcined by air to obtain g-C 3 N 4 Photocatalyst particles. Weigh 3g g-C 3 N 4 Disperse photocatalyst particles in 60mL hydrochloric acid solution, stir at room temperature for 3-5h, then wash with deionized water until neutral, the light yellow g-C 3 N 4 The photocatalyst particles were dried in an oven to obtain protonated g-C 3 N 4 catalyst of light.
[0051] Step 2: Weigh 0.03g of protonated g-C 3 N 4 Dissolve photocatalyst and 4.78g bismuth nitrate in 100mL deionized water, add 9mL acetic acid solution during stirring, after stirring at room temperature, add 1.17g potassium bromide and 3mmol sodium acetate to the solution, continue to stir for 2 hours, then carry out aging After reacting for 1 hour, the resulting precipitate was washed three times with deionized water and absolute ethanol, and then centrifuged and dried to obtain t...
Embodiment 2
[0053] Step 1: Put g-C 3 N 4 The precursor (urea) was moved into a semi-closed alumina crucible, placed in a muffle furnace and calcined by air to obtain g-C 3 N 4 Photocatalyst particles. Weigh 3g g-C 3 N 4 Disperse photocatalyst particles in 60mL hydrochloric acid solution, stir at room temperature for 3-5h, then wash with deionized water until neutral, the light yellow g-C 3 N 4 The photocatalyst particles were dried in an oven to obtain protonated g-C 3 N 4 catalyst of light.
[0054] Step 2: Weigh 0.1 g of protonated g-C 3 N 4 Dissolve photocatalyst and 3.19g bismuth nitrate in 100mL deionized water, add 9mL acetic acid solution during stirring, after stirring at room temperature, add 0.78g potassium bromide and 3mmol sodium acetate to the solution, continue stirring for 2 hours, then carry out aging After reacting for 1 hour, the resulting precipitate was washed three times with deionized water and absolute ethanol, and then centrifuged and dried to obtain the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com