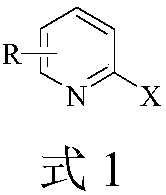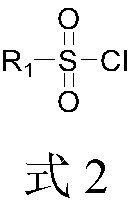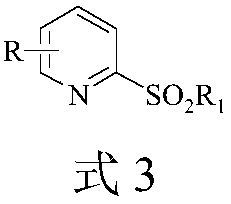Ultrasonic-assisted green synthetic method for 2-sulfonyl pyridine derivatives
A sulfonylpyridine and green synthesis technology, applied in organic chemistry and other fields, can solve the problems of residual transition metals in end products, expensive metal catalysts, increased production costs, etc., and achieve the effects of avoiding environmental problems, easy to obtain sources, and reducing reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1) 2-Benzenesulfonylpyridine:
[0051]
[0052] In a 50mL round-bottomed flask, add 1.14g of 2-chloropyridine, 1.94g of benzenesulfonyl chloride, 1.51g of sodium sulfite, and 15ml of water in sequence, and react ultrasonically for 20 minutes in a 30W / 100KHz ultrasonic reaction device. The crude product of 2-benzenesulfonylpyridine was obtained by filtration, and the crude product was washed with 95% ethanol to obtain 2.12 g of the corresponding pure product, with a yield of 97%.
[0053] NMR data:
[0054] 1 H NMR (400MHz, CDCl 3 ):δ=8.68(d, J=4.0Hz, 1H), 8.20(d, J=7.4Hz, 2H), 7.91–7.94(m, 1H), 7.44–7.62(m, 4H);
[0055] 13 C NMR (100MHz, CDCl 3 ): δ=158.8, 150.4, 138.7, 138.1, 133.7, 129.1, 128.8, 126.9, 122.1.
[0056] Control group 1
[0057] Replace ultrasonic-assisted reactions with room temperature stirred reactions:
[0058] In a 50mL round-bottomed flask, add 1.14g of 2-chloropyridine, 1.94g of benzenesulfonyl chloride, 1.51g of sodium sulfite, and 15...
Embodiment 2
[0084] In a 50mL round-bottomed flask, add 1.58g of 2-bromopyridine, 1.94g of benzenesulfonyl chloride, 1.51g of sodium sulfite, and 15ml of water in sequence, and react ultrasonically for 20 minutes in a 30W / 100KHz ultrasonic reaction device. The crude product of 2-benzenesulfonylpyridine was obtained by filtration, and the crude product was washed with 95% ethanol to obtain 2.14 g of pure 2-benzenesulfonylpyridine, with a yield of 98%.
Embodiment 3
[0086] In a 50mL round bottom flask, add 2.05g of 2-iodopyridine, 1.94g of benzenesulfonyl chloride, 1.51g of sodium sulfite, and 15ml of water in sequence, and react ultrasonically for 20 minutes in a 30W / 100KHz ultrasonic reaction device. The crude product of 2-benzenesulfonylpyridine was obtained by filtration, and the crude product was washed with 95% ethanol to obtain 2.14 g of pure 2-benzenesulfonylpyridine, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com