A kind of dye compound and its preparation method and use
A compound and enzyme synthesis technology, applied in the field of dye compounds, can solve the problems of high production cost, limited content, high biological toxicity, etc., and achieve the effects of operation process control, low production cost and easy operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
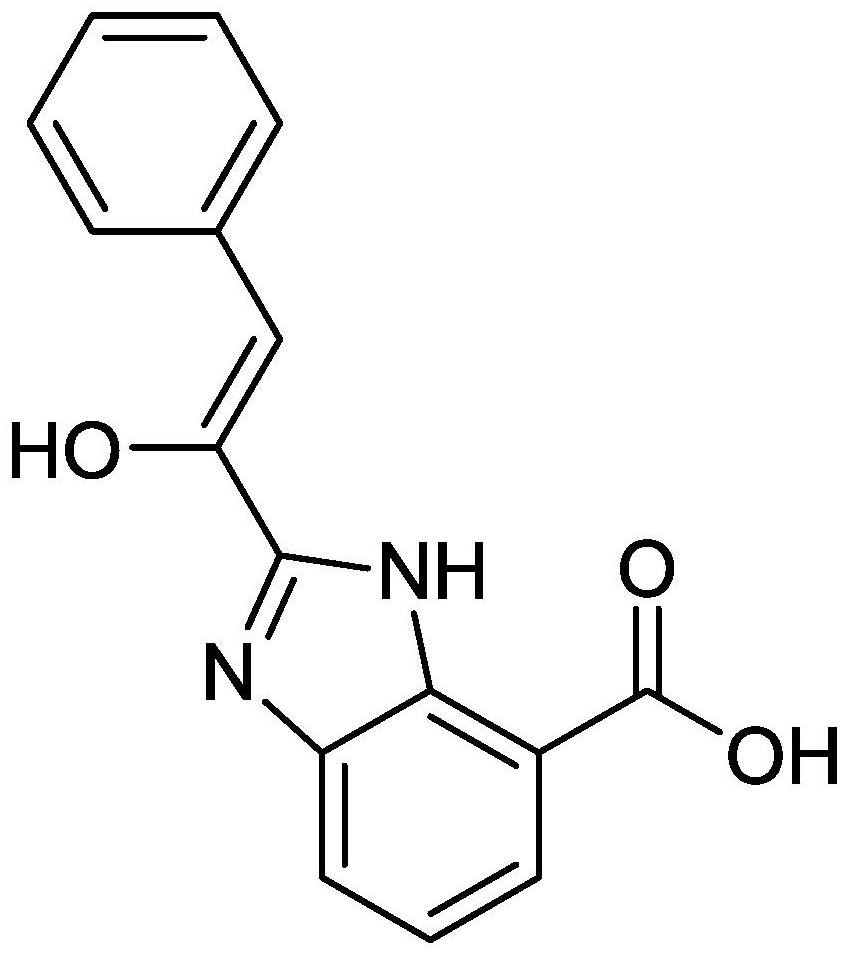
[0044] The preparation method of 2-phenylacetyl-benzimidazole-7-carboxylic acid.
[0045] (1) Experimental materials
[0046] Fusarium graminearum PH-1 was purchased from the American Standard Biological Collection (ATCC), and its number was ATCC MYA-4620; the fungal genome DNA extraction kit and pYES2 plasmid vector were purchased from Thermo Fisher Scientific (China ) Co., Ltd.; restriction endonucleases BamHI and EcoRI were purchased from Bao Biological Engineering (Dalian) Co., Ltd.; primers A1 and A2 were ordered from Sangon Bioengineering (Shanghai) Co., Ltd.
[0047] (2) Preparation of engineering bacteria S (Saccharomyces cerevisiae).
[0048](1) Purify the genomic DNA of Fusarium graminearum PH-1 of strain number ATCC MYA-4620 with a fungal genomic DNA extraction kit;
[0049] (2) Using the purified genomic DNA of Gibberella maize as a template, use primer A1: 5'-CGGGATCCCCTCAGCGCCTGTACAAAGACAC-3' and primer A2: 5'-CGGAATTCCTAGTCATCCTGTAAGCTGATGGTC-3' to amplify 2-p...
Embodiment 2
[0074] Verification of the StBI gene enzyme for the synthesis of 2-phenylacetyl-benzimidazole-7-carboxylic acid.
[0075] 1. Transform the blank plasmid pYES2 without the inserted enzyme gene StBI into Saccharomyces cerevisiae engineering strain INVSc1, cultivate it in the same way as in Example 1 for 72 hours, and take the fermentation broth for later use.
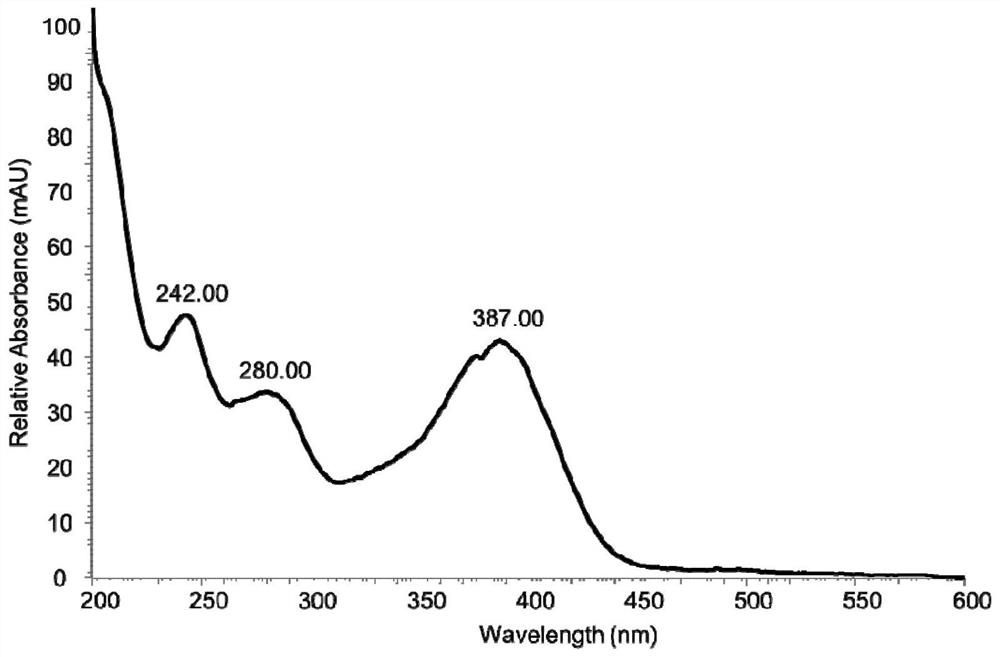
[0076] 2. Get the fermentation liquid of the engineering bacteria S (Saccharomyces cerevisiae) of Example 1 and the above-mentioned fermentation liquid, and carry out liquid phase detection, 380nm wavelength, and the elution method is gradient elution for 35min, 30-90% (acetonitrile: water).
[0077] The result is as Figure 4 Shown, the fermented liquid that does not have the blank plasmid pYES2 of insertion enzyme gene StBI to be transformed into Saccharomyces cerevisiae engineering bacterium INVSc1 is shown in (I), shows that 2-phenylacetyl-benzimidazole-7-carboxylic acid is not detected; The Saccharomyces cerevisiae ...
Embodiment 3
[0079] Engineering bacteria S (Saccharomyces cerevisiae) prepared by the method of Example 1, as the preparation method of 2-phenylacetyl-benzimidazole-7-carboxylic acid in Example 1, engineering bacteria S (Saccharomyces cerevisiae) was fermented and cultivated for 12 hours respectively Hours, 24 hours, 36 hours, 48 hours, 60 hours, 72 hours, 84 hours, 96 hours, 108 hours, 120 hours, as a result, 0.31g to 1.98g of 2 -Phenylacetyl-benzimidazole-7-carboxylic acid, such as Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com