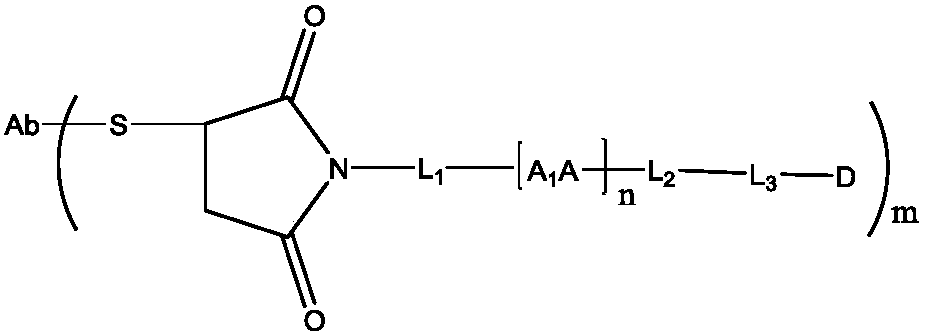
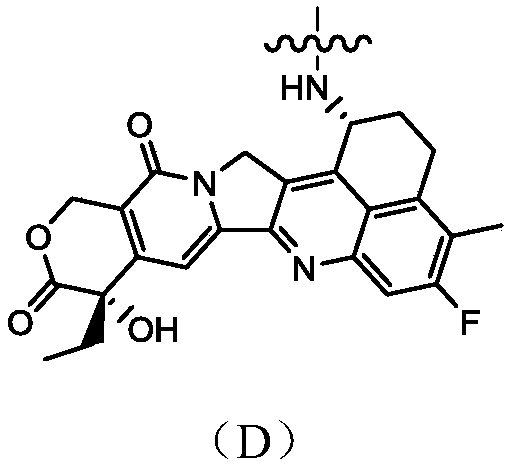
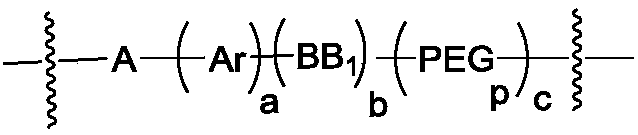Camptothecin-antibody conjugate
A technology of antibody conjugates and camptothecin, which can be used in anti-inflammatory agents, drug combinations, anti-tumor drugs, etc., and can solve the problems of insufficient hydrophilicity of molecules and the reduction of the efficacy of exitecan ADC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of compound 1
[0067]
[0068] 6-(maleimido) hexanoic acid succinimide ester (MC-OSu, 30g, 0.097mol) was dissolved in 200ml of dichloromethane, then added propargylamine (5.9g, 0.1mol), DIEA ( 24mL, 0.146mol), the reaction was stirred at room temperature. TLC (PE:EA=1:1) monitoring. After the reaction is complete, filter and collect the filtrate, add 200 mL of purified water to the filtrate, then dissolve it with 1 mol / L hydrochloric acid in water to adjust the pH to 5-6, separate the layers, wash the dichloromethane layer with saturated NaCl until neutral, and store at 40°C Concentrate under reduced pressure to obtain a crude product. The crude product was subjected to silica gel column chromatography (eluent PE / EA=3 / 1~2 / 1~1 / 1) to obtain 7.5 g of pure white solid. LC-MSm / z(ES + ): 249.1(M+H) +
Embodiment 2
[0070] Synthesis of compound 2
[0071]
[0072] Compound 1 (7g, 0.028mol), azide polyethylene glycol carboxyl (N 3 -PEG 8 -COOH, 6.62g, 0.014mol), cuprous iodide (3.37g) were dissolved in 200mL of dichloromethane, DIEA (2.8mL, 0.017mol) was slowly added dropwise at room temperature, and the reaction at room temperature was completed, TLC (DCM:MeOH= 8: 1) Monitor progress. After treatment, filtration, the filtrate was concentrated under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography (eluent DCM / MeOH=100 / 1-50 / 1-10:1) to obtain 9 g of the product. LC-MS m / z (ES + ): 716.4 (M+H) +
Embodiment 3
[0074] Synthesis of Compound 3
[0075]
[0076] Compound 1 (7g, 0.028mol), azide polyethylene glycol carboxyl (N 3 -PEG 4 -COOH, 4.07g, 0.014mol), cuprous iodide (3.37g) were dissolved in 200mL of dichloromethane, and DIEA (2.8mL, 0.017mol) was slowly added dropwise at room temperature, and the reaction at room temperature was completed, TLC (DCM:MeOH= 8: 1) Monitor progress. After treatment, filtration, the filtrate was concentrated under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography (eluent DCM / MeOH=100 / 1-50 / 1-10:1) to obtain 7.7 g of the product. LC-MS m / z (ES + ): 539.1(M+H) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com