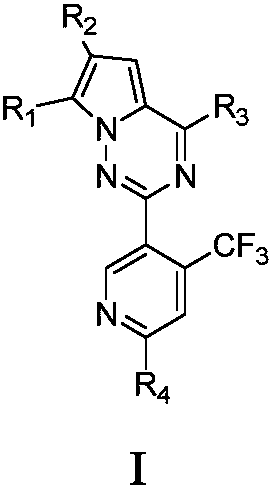
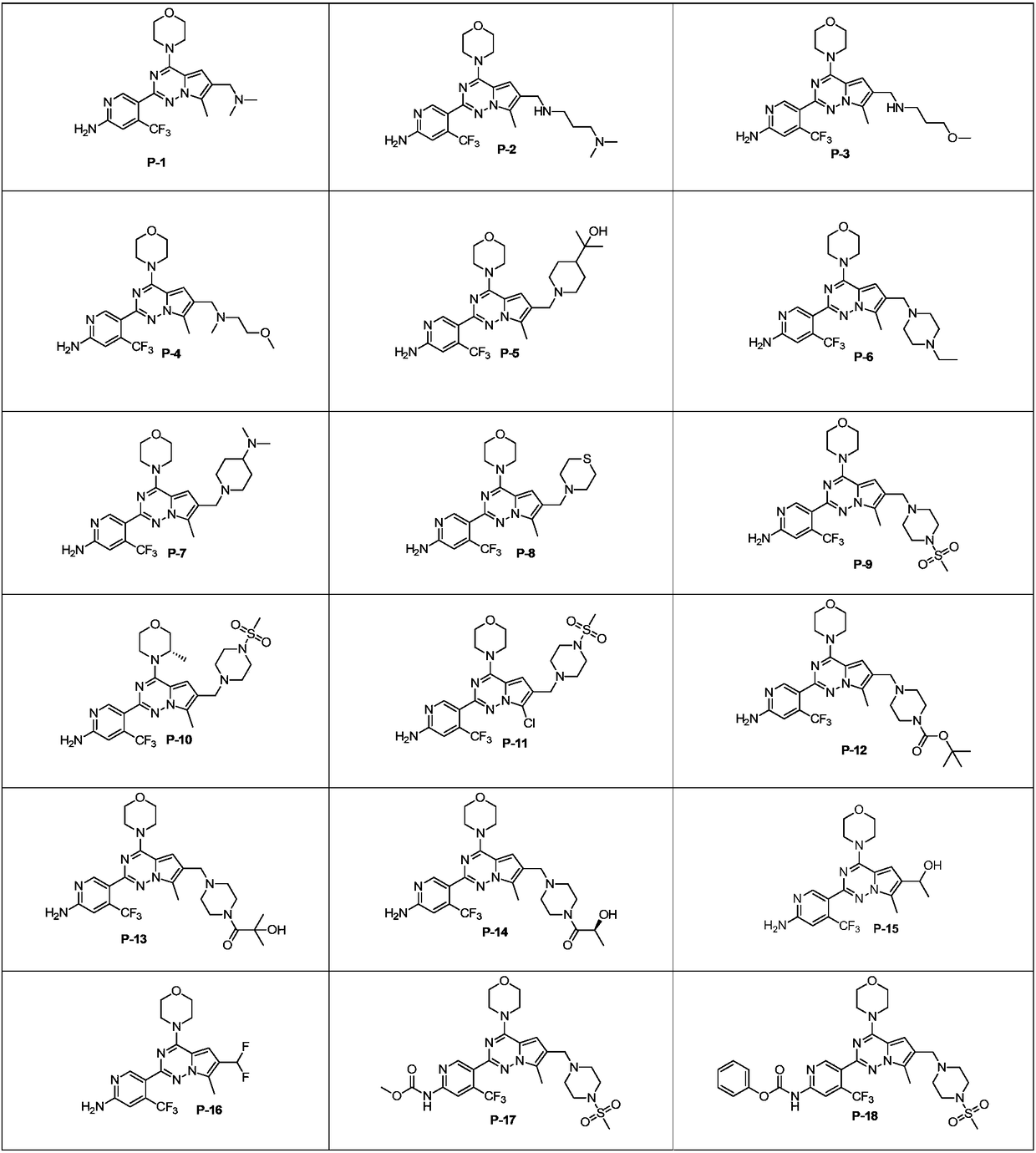
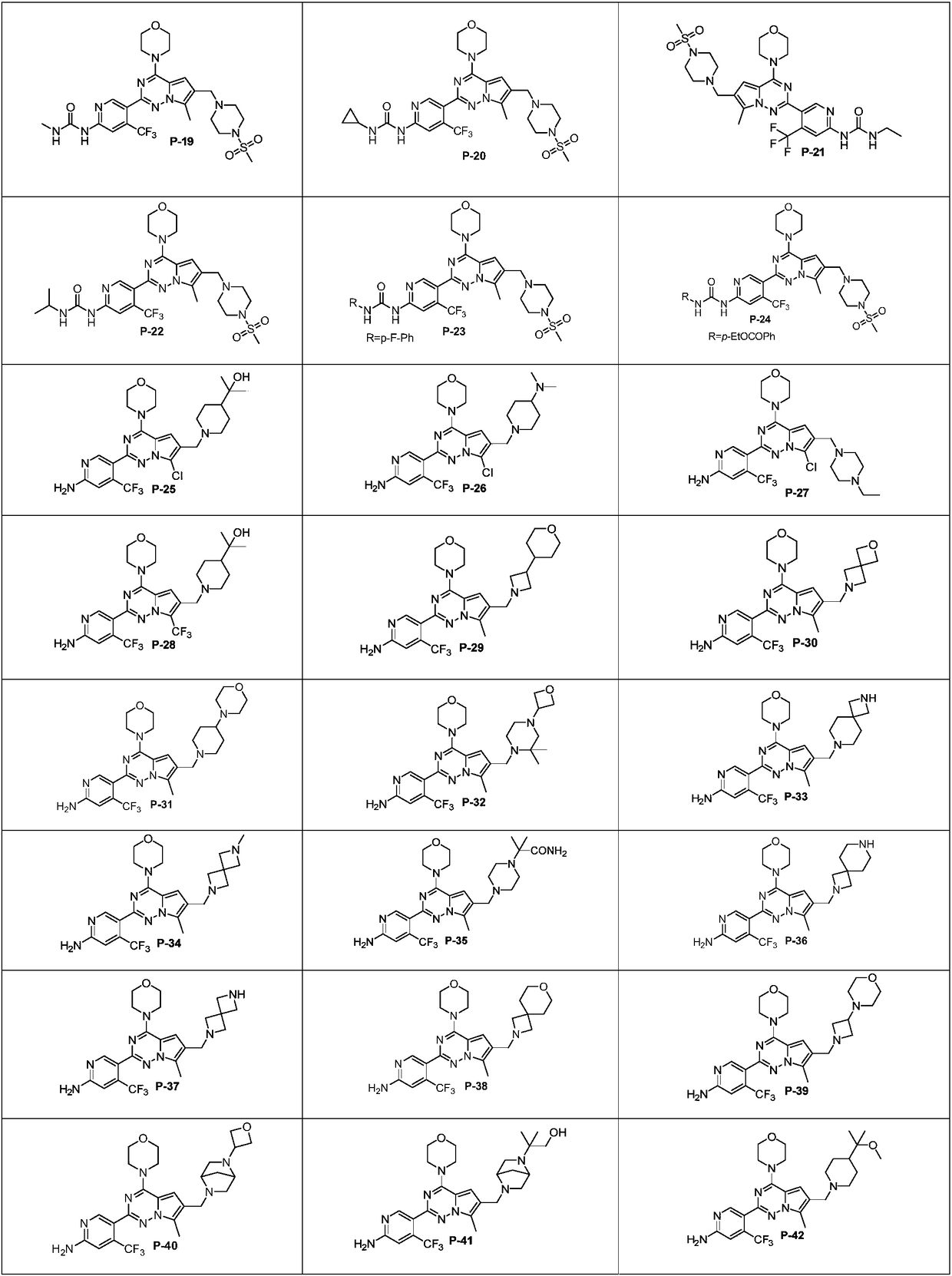
7-site substituted pyrrolotriazine compounds or pharmaceutically available salt thereof, and preparation method and use thereof
A technology of triazines and compounds, which is applied in the field of medicinal chemistry, can solve the problems of reporting PI3Kδ inhibitory activity, the large difference in properties between the mother nucleus and pyrrolotriazine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] As used herein, "alkyl" refers to straight or branched chain alkyl groups.
[0058] "Substituted" as used herein refers to substituting one or more hydrogen atoms. The halogen herein may be selected from at least one of F, Cl, Br and I, preferably at least one of F and Cl.
[0059] The present invention will be further described below in conjunction with the examples, but these examples are absolutely not intended to limit the present invention. In all examples, 1 H NMR was recorded by BrucherAM-400 and GEMINI-300 nuclear magnetic resonance instruments, chemical shifts were expressed in δ (ppm); mass spectra were recorded by MAT-95 mass spectrometer; silica gel for separation was 200-300 mesh.
[0060] Step a: Preparation of 4-cyano-5-methyl-1H-pyrrole-2-carboxylic acid ethyl ester (Aa2')
[0061]
[0062] Under an ice bath, to a solution of compound Aa1' (1.0 g, 6.5 mmol) and DMF (1.3 mL) in anhydrous acetonitrile (20 mL) was added dropwise chlorosulfonyl isocyan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com