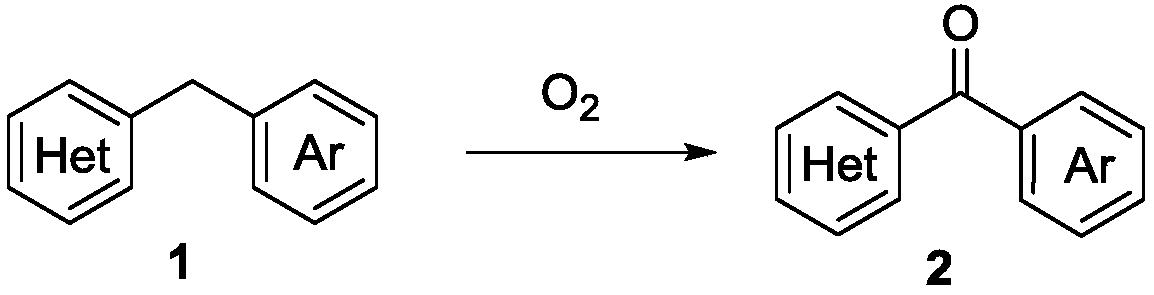
Method for simply and conveniently synthesizing heterocyclic aryl ketone compound
A technology for heterocyclic aryl ketones and compounds is applied in the field of organic synthesis and achieves the effects of low technical difficulty, few experimental steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0024] Under an oxygen atmosphere, 0.3 mmol of benzyl heterocycles 1a-1h and 2 mL of dimethyl sulfoxide were sequentially added to a Schlenk reaction tube, heated to 130° C. in an IKA (constant heating magnetic stirrer), and stirred for 48 h. After the reaction was completed, it was cooled to room temperature and quenched with distilled water. It was then extracted with ethyl acetate (3 x 20 mL). The organic layers were combined, and the organic phase was dried over anhydrous sodium sulfate. The pure target products 2a-2h were obtained by column chromatography.
Embodiment 1
[0026]
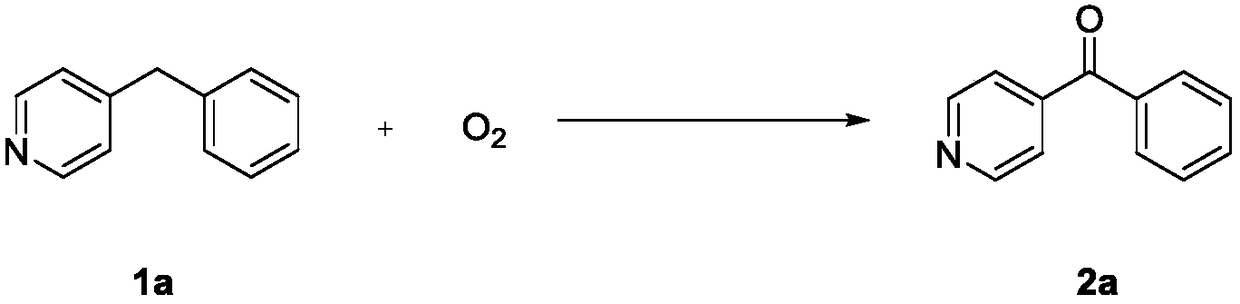
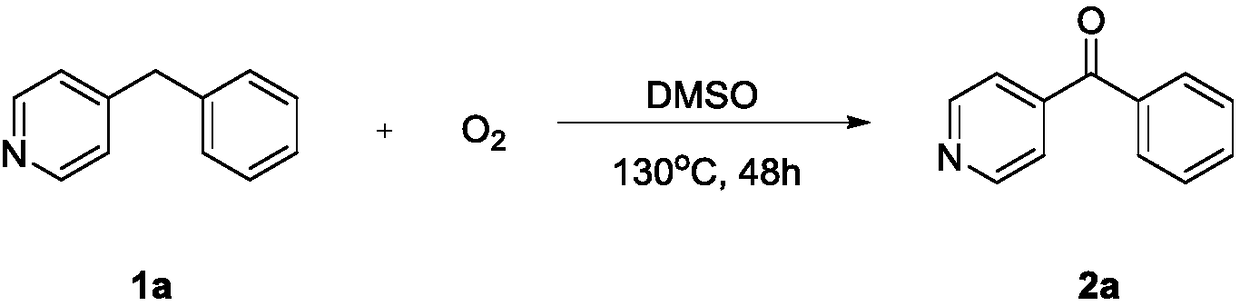
[0027] Under an oxygen atmosphere, 0.3 mmol of 4-benzylpyridine (1a) and 2 mL of dimethyl sulfoxide were sequentially added to a Schlenk reaction tube, heated to 130° C. in an IKA (constant heating magnetic stirrer), and stirred for 48 h. After the reaction was completed, it was cooled to room temperature and quenched with distilled water. It was then extracted with ethyl acetate (3 x 20 mL). The organic layers were combined, and the organic phase was dried over anhydrous sodium sulfate. The pure target product 2a (47.8 mg, 87%) was obtained by column chromatography. The characterization data of this compound are as follows: 1 HNMR (600MHz, CDCl 3 ):8.82(d,J=6.0Hz,2H),7.82(dd,J=12.0,6.0Hz,2H),7.65(td,J=9.0,6.0Hz,1H),7.59(dd,J=6.0, 6.0Hz, 2H), 7.52(td, J=9.0, 6.0Hz, 2H); 13 C NMR (101MHz, CDCl 3 ): 195.1, 150.4, 144.3, 135.9, 133.5, 130.1, 128.6, 122.8.
Embodiment 2
[0029]
[0030] Under an oxygen atmosphere, 0.3 mmol 2-benzylpyridine (1b) and 2 mL dimethyl sulfoxide were sequentially added to a Schlenk reaction tube, heated to 140° C. in an IKA (constant heating magnetic stirrer), and stirred for 48 h. After the reaction was completed, it was cooled to room temperature and quenched with distilled water. It was then extracted with ethyl acetate (3 x 20 mL). The organic layers were combined, and the organic phase was dried over anhydrous sodium sulfate. The pure target product 2b (42.8 mg, 78%) was obtained by column chromatography. The characterization data of this compound are as follows: 1 HNMR (600MHz, CDCl 3 ): 8.60(dd, J=6.0,6.0Hz,1H),7.96(d,J=12.0Hz,2H),7.91(dd,J=12.0,6.0Hz,1H),7.76(td,J=9.0, 6.0Hz, 1H), 7.47(td, J=9.0, 6.0Hz, 1H), 7.38-7.34(m, 3H); 13 C NMR (101MHz, CDCl 3 ): 193.8, 155.1, 148.5, 137.0, 136.3, 132.9, 131.0, 128.1, 126.2, 124.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com