Method for N-methylation reaction of nitro-compound
A technology for nitro compounds and aryl nitro compounds is applied in the preparation of amino compounds, the preparation of organic compounds, the preparation of amino hydroxy compounds, etc. Strong Interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
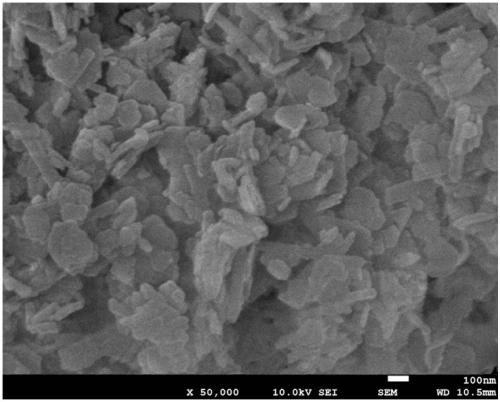
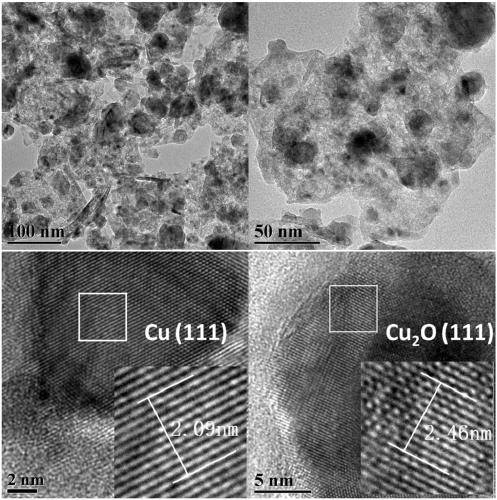
[0046] Embodiment 1: take hydrotalcite as the preparation of the Cu-based catalyst of precursor
[0047] Cu(NO 3 ) 2 ·3H 2 O, Al(NO 3 ) 3 9H 2 O is formulated with a molar ratio of 2:1 so that the total concentration of metal ions is 1molL -1 Mixed metal salt solution; NaOH, Na 2 CO 3 Formulated with a molar ratio of 5:1 to prepare a total concentration of Na ions of 1.2molL -1 precipitant solution. Use a dropping funnel to add the metal salt solution and the precipitant solution dropwise into deionized water (the same volume as the metal mixed salt solution), and precipitate under stirring conditions at room temperature. By controlling the drop rate of the metal salt and the precipitant, the system The pH was maintained at 10.0 ± 0.2. After the dropwise addition, it was transferred to an oil bath at 60°C for aging for 15 hours, and the color of the system gradually changed from blue to dark gray. After aging, filter while hot, then wash and filter with deionized wa...
Embodiment 2
[0050] Embodiment 2: Cu-based catalysts catalyze the N-methylation reaction of nitro compounds
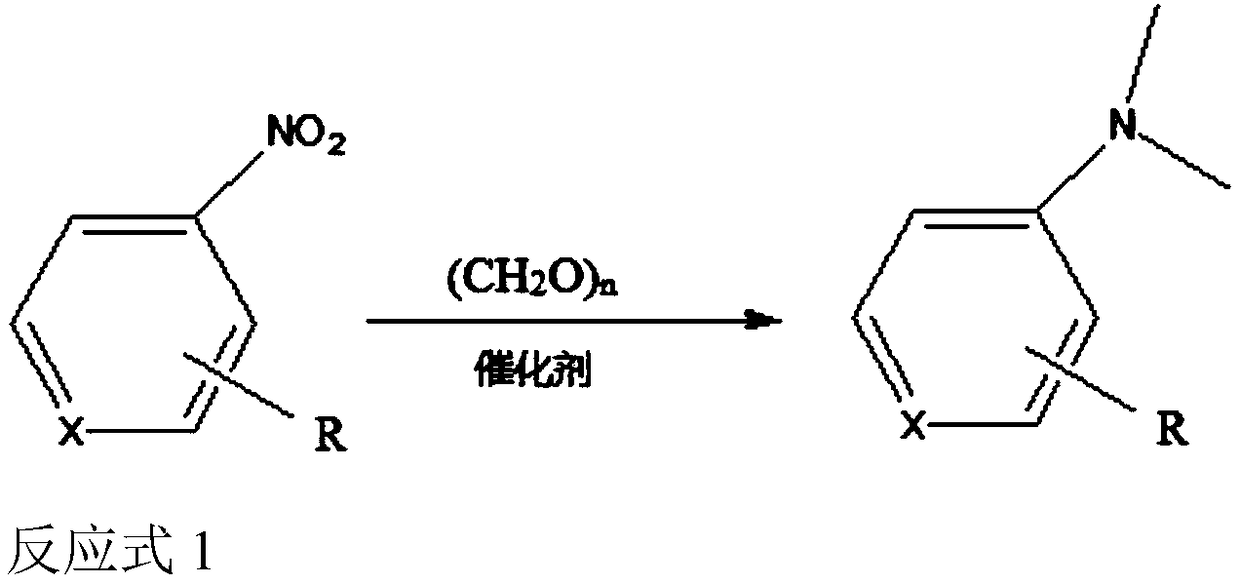
[0051] Add 6mg of the Cu catalyst prepared in Example 1 to a thick-walled glass reaction tube, then add 0.25mmol p-nitrotoluene, 150mg paraformaldehyde, 0.5mmol NaCO 3 and 3 mL of solvent H 2 O / DMSO=1:1, the reaction tube was placed in an oil bath with stirring at 130°C for 15 hours, cooled to room temperature, and analyzed by gas chromatography. The results showed that the conversion rate of p-nitrotoluene was 100%, and the selectivity of the product p-methyl N,N-dimethylaniline was 100%.
Embodiment 3 to 10
[0053] Same as Example 2 operation steps, change the kind of aromatic nitro compound (i.e. substrate), in the presence of the Cu-based catalyst prepared in Example 1, complete the N-methylation reaction of other aromatic nitro compounds, each The yields of substrates to generate corresponding methylated products are shown in Table 1:
[0054] Table 1
[0055]
[0056] As can be seen from the results of Examples 1 to 10, the method for the direct N-methylation reaction of nitro compounds according to the present invention can effectively convert various nitro compound substrates into N-methyl products, and the method according to the present invention is cost-effective and beneficial to large-scale industrial production.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com