Cyanodiphenylethylene compound with stable Z/E configuration in excited state and preparation method and application thereof
A technology for cyanodiphenylethylene and compounds, which is applied in the field of cyanodiphenylethylene compounds, can solve the problems of small steric hindrance, difficulty in obtaining Z/E isomers, etc., and achieves the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
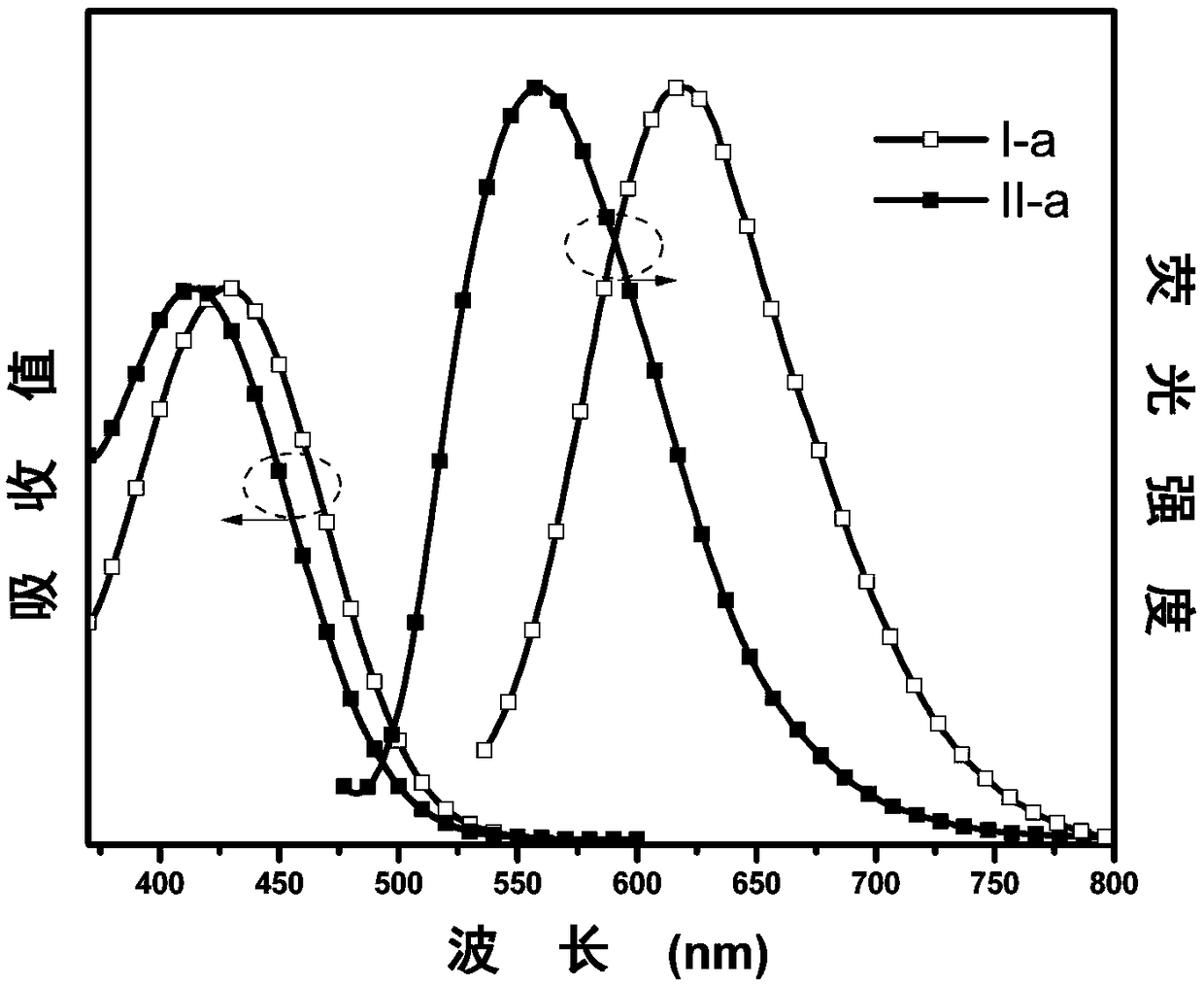
[0057] The synthesis of embodiment 1 I-a and II-a
[0058] (1) Under nitrogen protection, phenoxazine (V-a, 1.83g, 10mmol), 4-bromophenylacetonitrile (V-b, 2.28g, 12mmol), palladium acetate (0.13g, 0.6mmol), three (tert-butyl ) Phosphine (0.53g, 2.6mmol) and sodium tert-butoxide (2.50g, 26mmol) were dissolved in toluene (30mL), and the system was heated to 115°C for reflux for 24 hours. After the system was cooled, it was extracted with deionized water and dichloromethane, and the obtained organic phase was added with anhydrous MgSO 4 After drying, concentrate under reduced pressure, then separate and purify by column chromatography, the stationary phase is 300-400 mesh silica gel, the mobile phase is dichloromethane / petroleum ether (volume ratio 1:3), and finally the intermediate product V-c is obtained as a white solid 1.48 g, 50% yield. 1 H NMR (500MHz, CDCl 3 )δ (TMS, ppm) 7.59 (d, J = 8.4Hz, 2H), 7.40 (d, J = 8.2Hz, 2H), 6.74-6.57 (m, 6H), 5.90 (d, J = 7.6Hz, 2H ), 3....
Embodiment 2
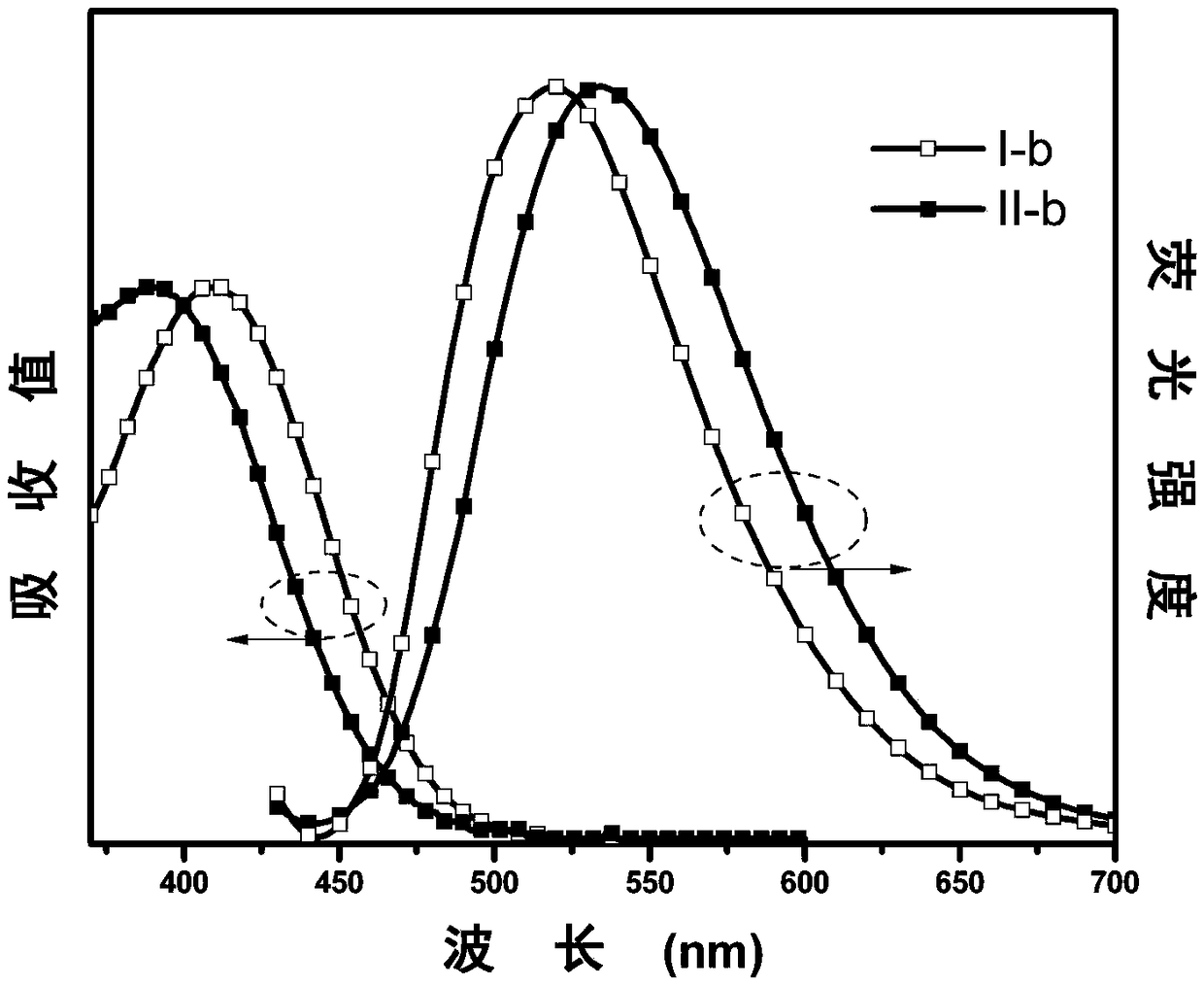
[0062] The synthesis of embodiment 2 I-b and II-b
[0063] The synthetic method is the same as in Example 1, except that in step (2), 4-trifluoromethylbenzaldehyde is replaced by benzaldehyde (0.1g, 1mmol), and finally the target product I-b (0.32g, yield 83 %), II-b (0.02 g, yield 5%). The characterization structure of the confirmed substance is as follows:
[0064] I-b: 1 H NMR (500MHz, CDCl 3 )δ(TMS,ppm)7.90-7.99(m,4H),7.64(s,1H),7.49-7.56(m,3H),7.47(d,J=8.5Hz,2H),6.73(dd,J= 8.5Hz, J=1.7Hz, 2H), 6.66 (dt, J=15.2Hz, J=7.3Hz, 4H), 5.98 (dd, J=7.9Hz, J=1.4Hz, 2H). MALDI-TOF MS theoretical value (C 27 h 18 N 2 (0) m / z: 386.4, found: 387.4.
[0065] II-b: 1 H NMR (500MHz, CDCl 3 )δ (TMS, ppm) 7.62 (d, J = 8.4Hz, 2H), 7.48 (s, 1H), 7.33-7.40 (m, 3H), 7.30 (t, J = 7.4Hz, 2H), 7.24 (d , J=7.3Hz, 2H), 6.59-6.77 (m, 6H), 5.97 (d, J=7.8Hz, 2H). MALDI-TOF MS theoretical value (C 27 h 18 N 2 (0) m / z: 386.4, found: 387.4.
Embodiment 3
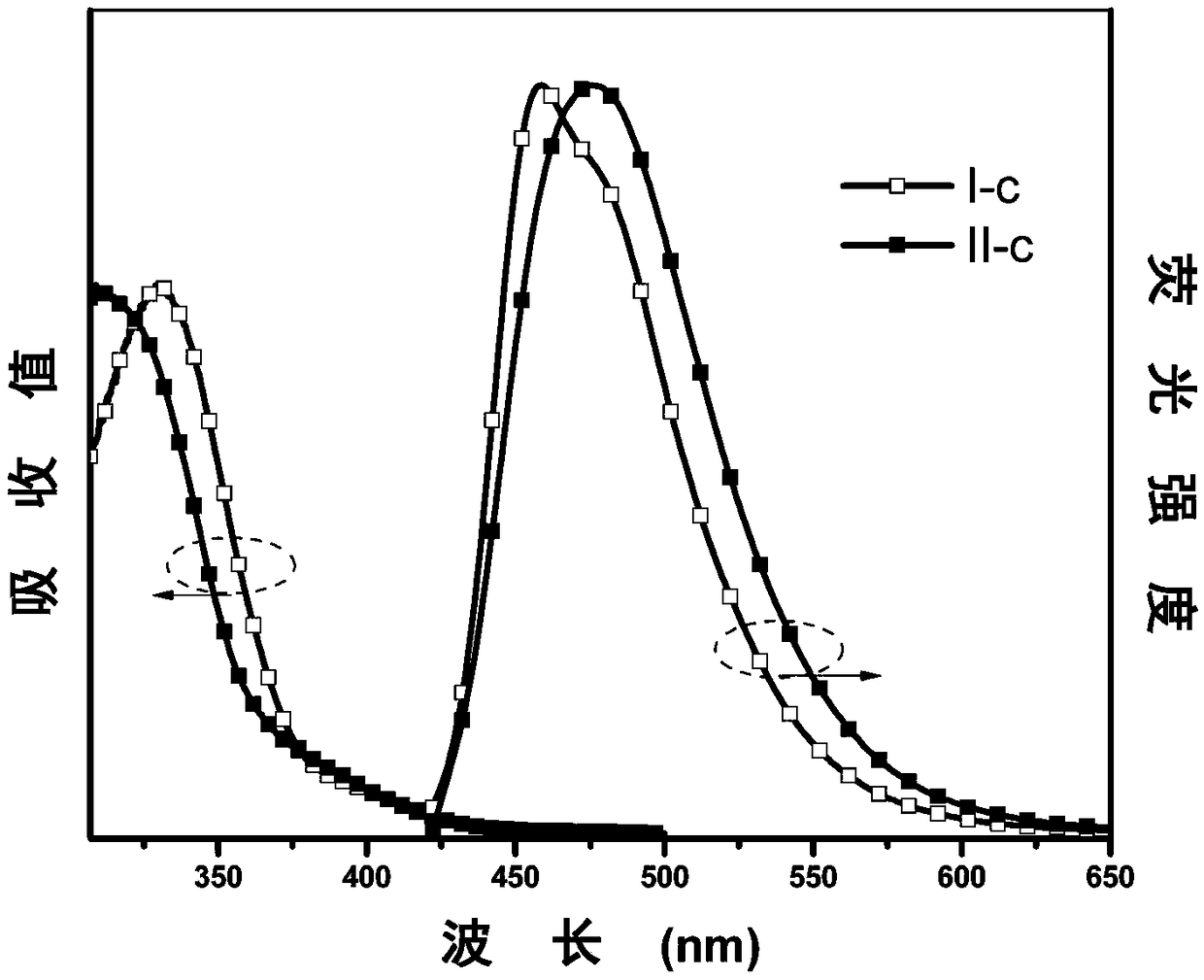
[0066] The synthesis of embodiment 3 I-c and II-c
[0067] (1) Under nitrogen protection, phenoxazine (V-a, 1.83g, 10mmol), p-bromoiodobenzene (3.39g, 12mmol), cuprous iodide (0.04g, 0.22mmol), sodium tert-butoxide (2.11 g, 22mmol) and cyclohexanediamine (0.13g, 1.1mmol), were dissolved in ultra-dry 1,4-dioxane (30ml), and the system was heated to 110°C and refluxed for 6 hours. After the system was cooled, it was extracted with deionized water and dichloromethane, and the obtained organic phase was added with anhydrous MgSO 4 After drying, it was concentrated under reduced pressure, and then separated and purified by column chromatography. The stationary phase was 300-400 mesh silica gel, and the mobile phase was dichloromethane / petroleum ether (volume ratio 1:9), and finally the intermediate product VI was obtained as a white solid. -a 2.27g, yield 67%. The characterization structure of the confirmed substance is as follows:
[0068] 1 H NMR (500MHz, CDCl 3 )δ (TMS, ppm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com