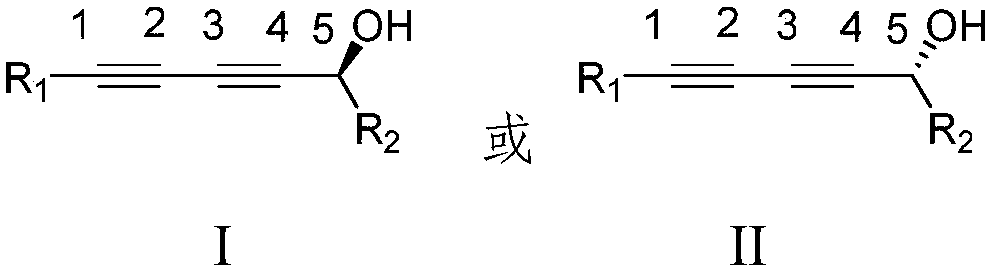
Fakalin alcohol compound enantiomer, method for synthesizing same and application of Fakalin alcohol compound enantiomer
An enantiomer and synthesis method technology, which is applied to the application field of anti-HeLa tumor cells, can solve the complex operation and processing procedures, the limited enantioselectivity of products in the scope of application, and the simple preparation and scale of fakarin alcohol compounds. Chemical production and other problems, to achieve the effects of high stereoselectivity, strong anti-HeLa tumor cell proliferation activity, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
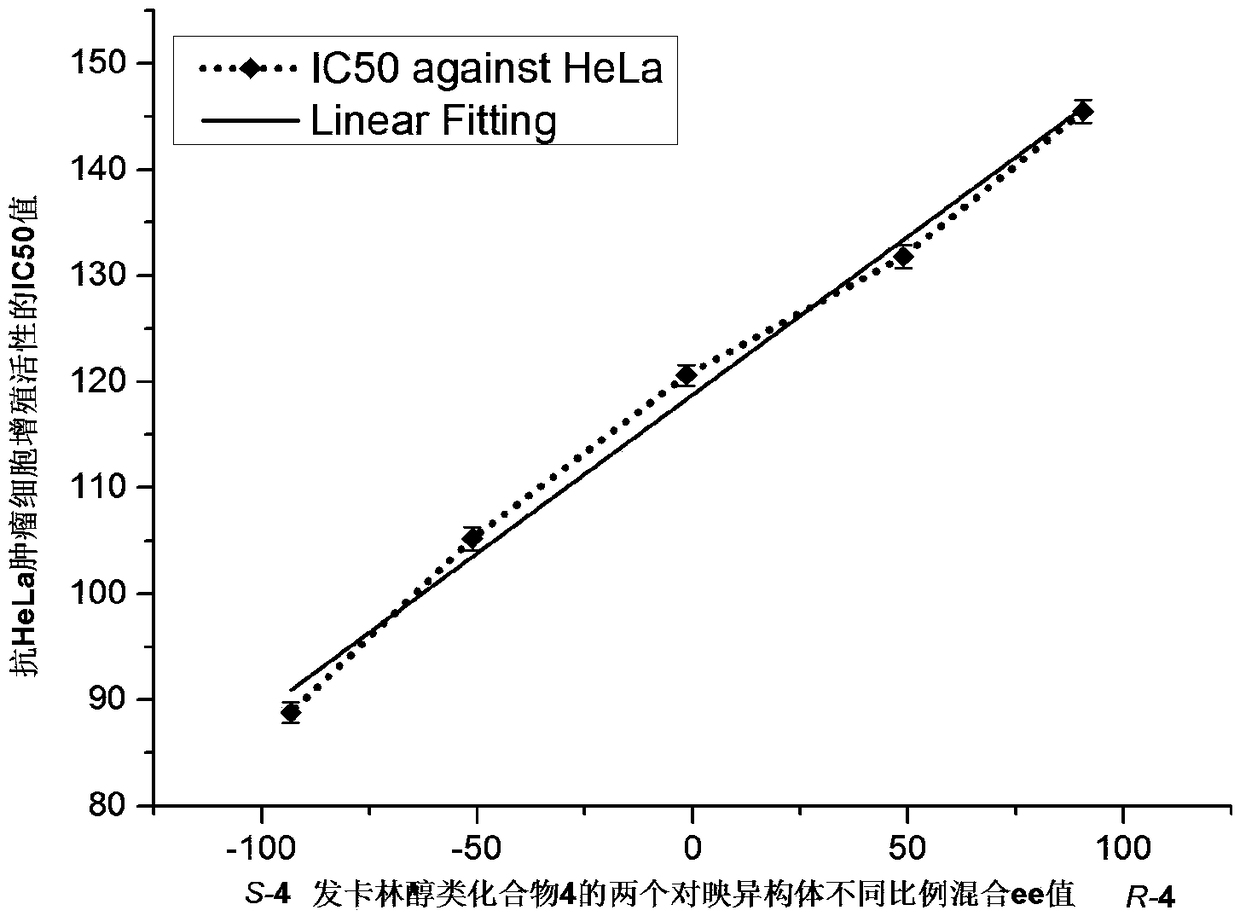
[0045] Example 1: (S)-1-(2-Bromophenyl)-5-(triisopropylsilyl)-pentane-2,4-diyn-1-ol and (R)-1-(2 Preparation of -bromophenyl)-5-(triisopropylsilyl)-pentane-2,4-diyn-1-ol, namely S-4 and R-4 (Entry 4)
[0046]1. Preparation of 1-(triisopropylsilyl)-1,3-butadiyne
[0047] At 0°C, 5 mL (97.6 mmol) of bromine was added dropwise to a saturated aqueous KOH solution and stirred for 30 minutes, then 10 mL (108.2 mmol) of 2-methyl-3-butyn-2-ol was added and stirring was continued for 30 minutes. The reaction mixture was extracted with ether, dried, evaporated to remove the solvent, and separated by silica gel column chromatography to obtain 4-bromo-2-methylbut-3-yn-2-ol as a yellow oily liquid for use in a yield of 90%. CuCl was added to the butylamine aqueous solution, 10 g of hydroxylamine hydrochloride and 40.3 g (220.8 mmol) of triisopropylsilyl acetylene were added, and the reaction was carried out. After the reaction was completed, it was cooled in an ice bath. After adding 30....
Embodiment 2
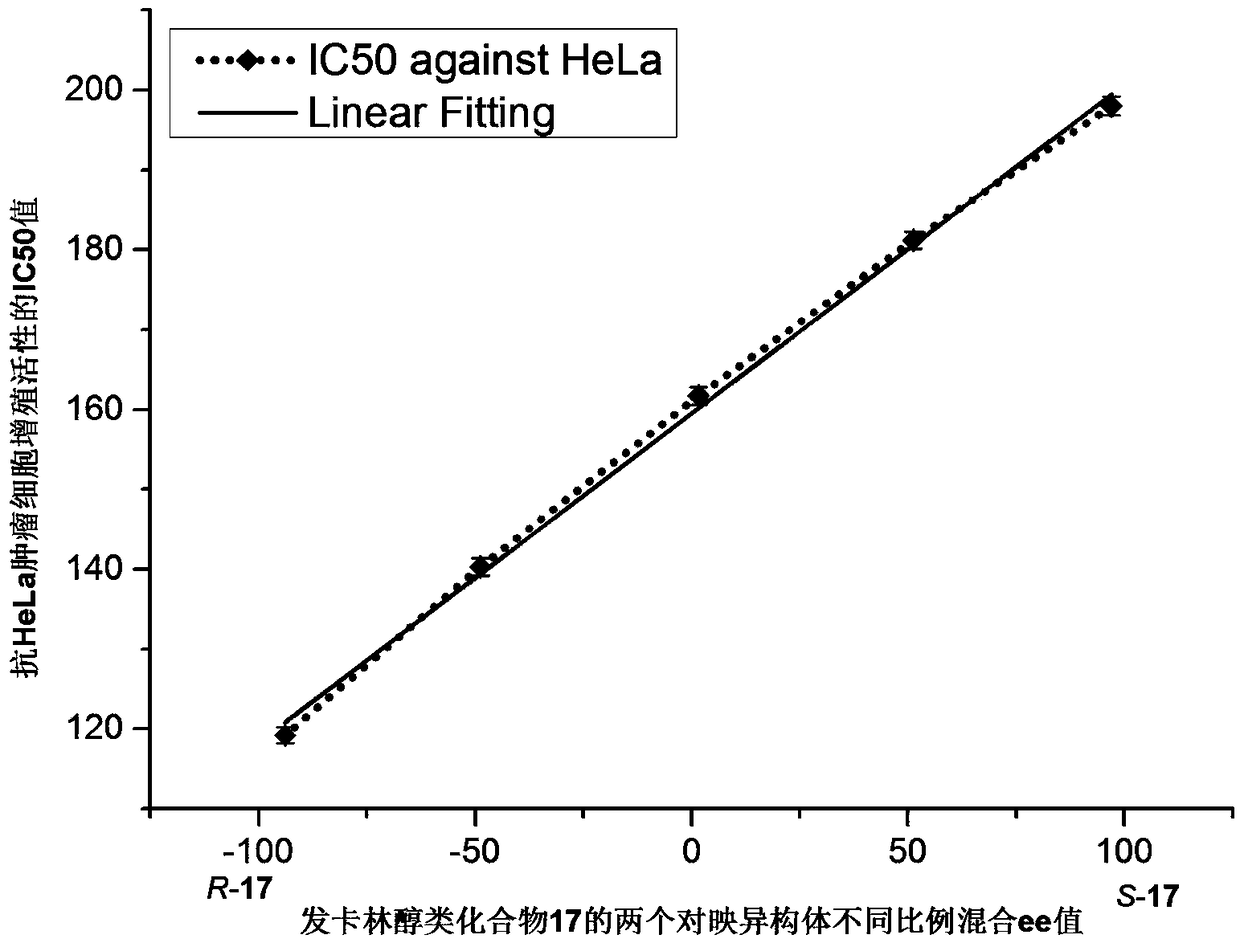
[0056] Example 2: (R)-1-(3-Methoxyphenyl)undecan-2,4-diyn-1-ol and (S)-1-(3-methoxyphenyl)undecane Preparation of alkane-2,4-diyn-1-ols, namely R-17 and S-17 (Entry17)
[0057] 1. Preparation of 1-(n-hexaneyl)-1,3-butadiyne
[0058] Same as the description in Example 1. The coupled alkyne described in Example 1 is 1-octyne, the yield is 89%, 1 H NMR (400MHz, CDCl3): δ2.26 (t, 2H, J=7.0Hz), 1.95 (s, 1H), 1.34 (m, 6H), 0.89 (t, 3H, J=6.6Hz), 1.54 ( p, 2H, J = 6.6 Hz).
[0059] 2. Preparation of R-17 and S-17
[0060] As described in Example 1, the added substrate was 3-methoxybenzaldehyde (1 equiv, 0.5 mmol), and the products R-17 and S-17 were obtained respectively in yields of 80% and 82%, and the products were detected by HPLC analysis , using a chiral Chiralcel OD column, the mobile phase n-hexane / isopropanol is 80 / 20, the flow rate is 0.9 mL / min, the detection wavelength is 254 nm, and the retention times are t 1 =5.5min, t 2 =7.2min, the enantioselectivity ee values...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com