Conjugate of triterpene and linear chain amino derivative and application of conjugate
A technology of straight-chain amino and derivatives, which can be applied to medical preparations containing active ingredients, steroids, organic chemistry, etc., and can solve the problems that the inhibitory effect of influenza virus has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
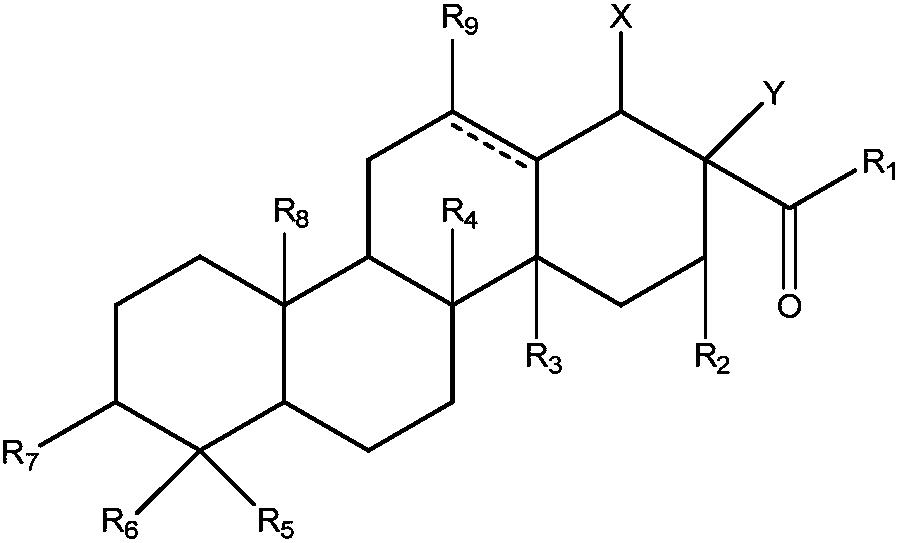
[0059] Embodiment 1: The preparation method of the conjugate of this triterpene and straight-chain amino derivatives can be prepared by the following technical routes:
[0060]
[0061] Wherein, compound M22 is synthesized as follows:
[0062]
[0063] Get 1g of oleanolic acid (OA) in a 50mL reaction bottle, dissolve it in 20mL DMF, add 845mg TBTU and 340mgDIEA, react at room temperature, TLC detection after 12h, developer ratio petroleum ether: ethyl acetate=3:1; Completely evaporate DMF to dryness, use water / ethyl acetate system (water:ethyl acetate volume ratio 1:1) to extract 3 times, take the organic phase in MgSO 4 After drying, the solvent was evaporated and recrystallized (ethanol:water=3:1); 1582 mg of white flocculent solid M38 was obtained with a yield of 92.8%, which was ready for use;
[0064] Take 1g of compound M38 in a 50mL reaction flask, dissolve it in 15mL of DMF, add 217mg of 4-aminobutanol, then add 1.2eq of sodium carbonate, react at room temperatu...
Embodiment 2
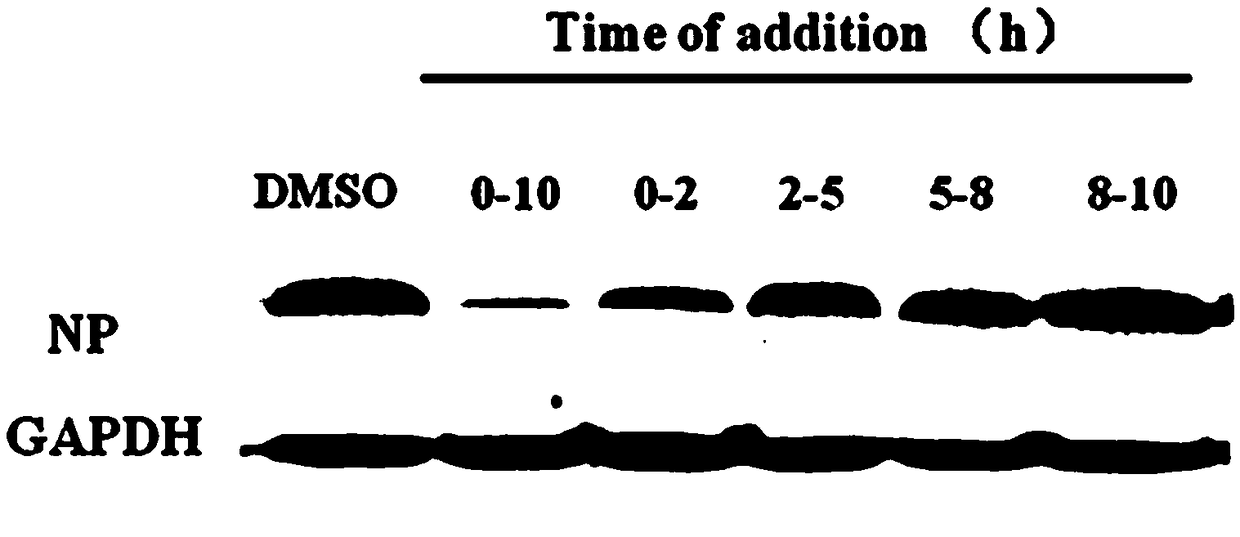
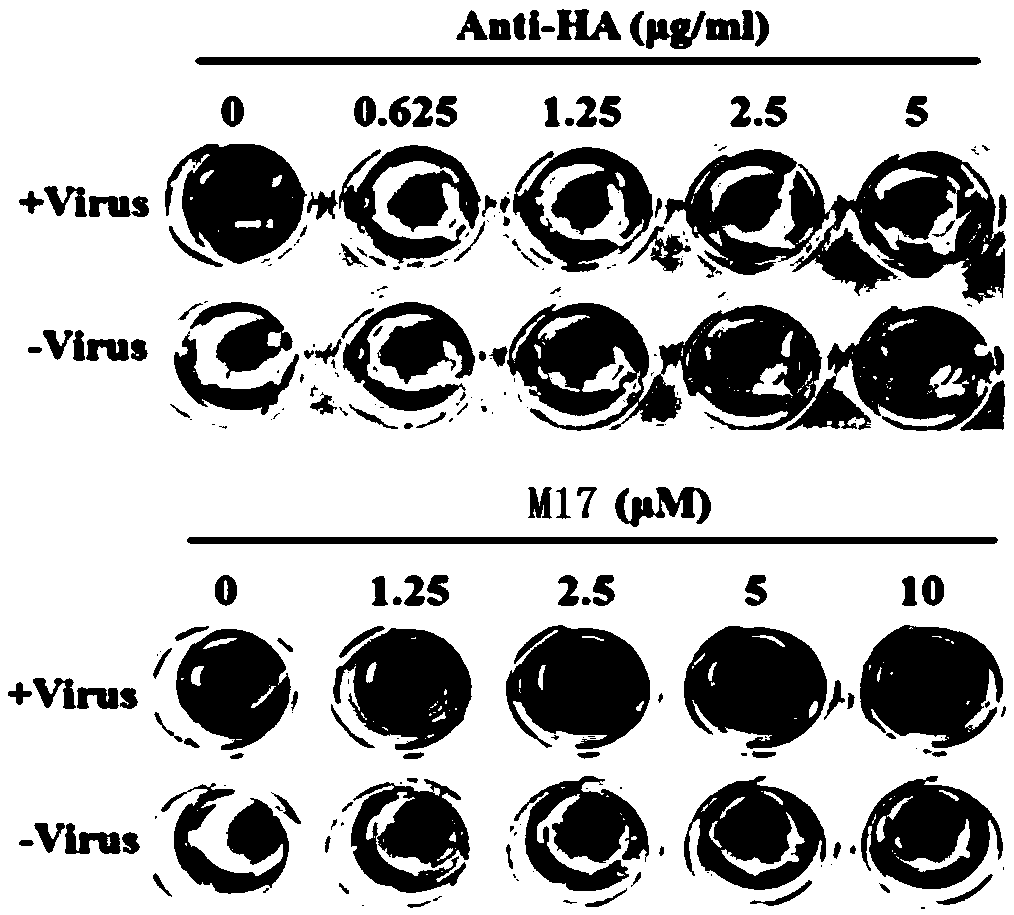
[0095] Embodiment 2: The compound of the present invention inhibits the biological activity evaluation method of influenza virus entering cells
[0096] 1. Cytopathic Efficacy (CPE) Inhibition Test
[0097] After influenza virus infects cells, it will cause cell pathology, which will reduce cell viability; if the drug can inhibit the replication of influenza virus, it will reduce the number of cell lesions and increase cell viability; specifically:
[0098] (1) Canine kidney epithelial cells (MDCK) were passaged into a white 96-well plate at a ratio of 1:3, and cultured in DMEM medium containing 10% FBS for 24 hours in a cell culture incubator at 37°C;
[0099] (2) Add influenza virus [A / WSN / 33 (H1N1), multiplicity of infection (MOI) = 1] and 100 μM / L of the compound to be tested to 100 μl of DMEM containing 2 μg / mL TPCK-treated trypsin and 1% FBS Mix well; the negative control of the compound is 1% DMSO (the solvent used for diluting the compound); set up a group of experime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com