Method for desorbing arsenic from arsenic-containing soot
A technology for removing arsenic dust and dust, which is applied in the field of secondary resource utilization of complex nonferrous metals, can solve problems such as arsenic loss, operating environment hazards, and hidden safety hazards, and achieve the effects of increasing added value, good arsenic removal effect, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
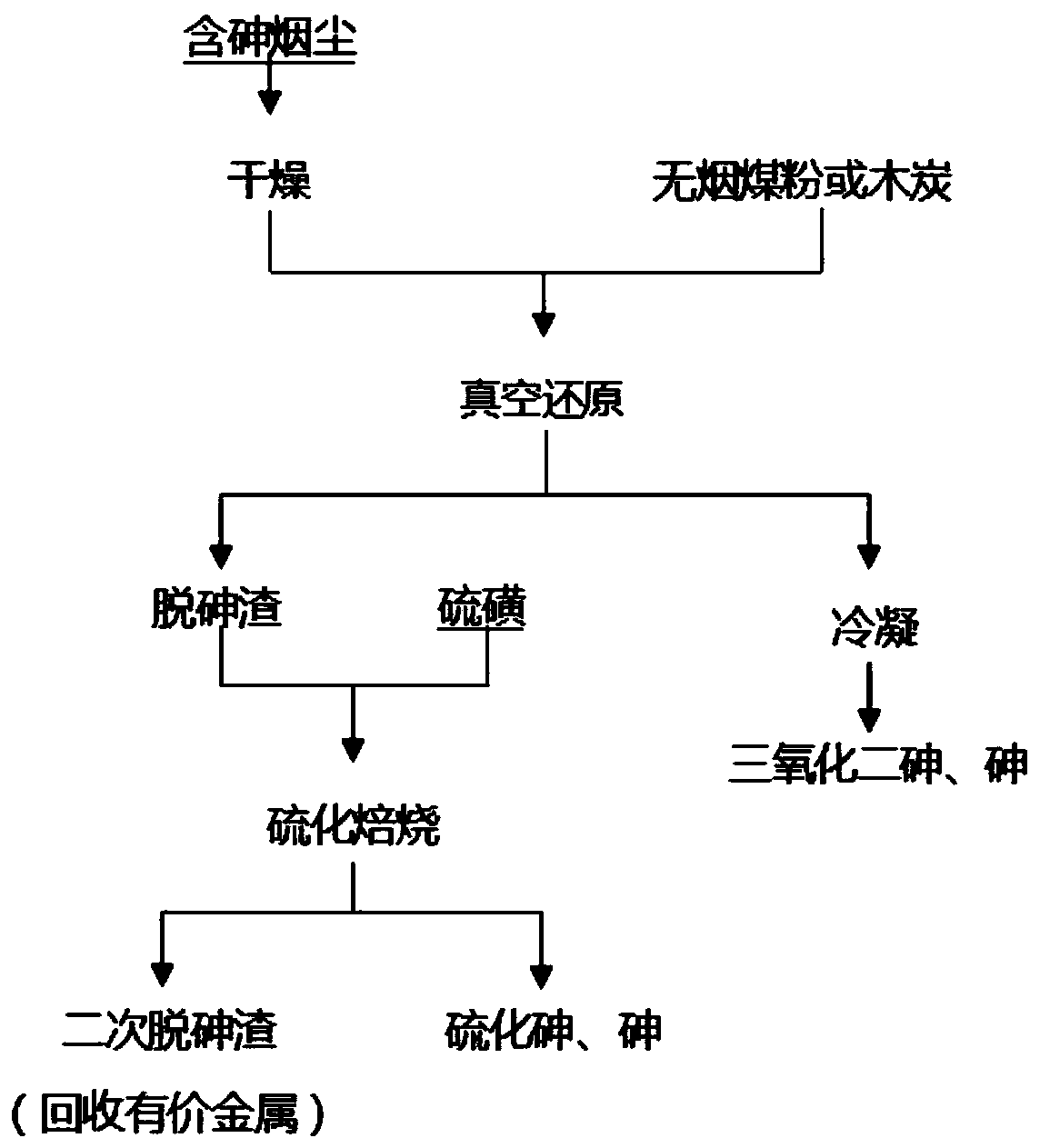
[0046] Grind 200g of arsenic-containing fumes (containing 11.99% of arsenic, 10.98% of lead, 12.11% of zinc, 4.75% of copper, and 3.08% of bismuth) into an electric blast drying oven, and dry at 260°C for 5 hours; take 100g of dried Mix arsenic-containing fumes with 10g of sieved anthracite coal evenly, and put them into a self-made vacuum reactor; after vacuuming to below 500Pa, heat the reactor to 700°C at a heating rate of 17°C / min and keep it warm for 3 hours. The pressure in the medium reactor fluctuates within the range of 10-260Pa, and the temperature of the condensation zone fluctuates between 127-153°C; after the reaction, the arsenic-removed slag and condensate are taken out, the arsenic-removed slag contains 5.75% of arsenic, and the arsenic removal rate is 71.71% , the condensate contains 98.47% arsenic; take 20g of arsenic-removed slag, grind it to below 100 mesh, add 2g of sulfur powder, mix it evenly, and place it in the reactor; vacuumize the reactor to 5Pa, kee...
Embodiment 2
[0048] Grind 1000g of arsenic-containing fumes (containing 14.83% of arsenic, 13.95% of lead, 11.02% of zinc, 3.73% of copper, and 4.26% of bismuth) into an electric blast drying oven, and dry at 230°C for 10 hours; take 100g of dried Mix arsenic-containing fumes with 20g of sieved anthracite coal evenly, and put them into a self-made vacuum reactor; after vacuuming to below 20Pa, heat the reactor to 1000°C at a heating rate of 15°C / min, keep it warm for 5 hours, and keep it warm. The pressure inside the medium reactor varies between 15-550Pa, and the temperature in the condensation zone fluctuates between 170-246°C; after the reaction, the arsenic-removed slag and condensate are taken out, the arsenic-removed slag contains 7.66% of arsenic, and the arsenic removal rate is 70.93% , the condensate contains 95.94% arsenic; take 20g of arsenic-removed slag, grind it to below 100 mesh, add 4g of sulfur powder, mix it evenly, and place it in the reactor; vacuumize the reactor to 40P...
Embodiment 3
[0050] Grind 400g of arsenic-containing fumes (containing 18.72% of arsenic, 12.33% of lead, 15.01% of zinc, 3.31% of copper, and 4.33% of bismuth) into an electric blast drying oven, and dry at 240°C for 7 hours; take 100g of dried Mix arsenic-containing fumes with 10g of charcoal powder evenly, and put them into a self-made vacuum reactor; after vacuuming to below 650Pa, heat the reactor to 800°C at a heating rate of 20°C / min, and keep it warm for 10 hours. The pressure fluctuated between 20-870Pa, and the temperature in the condensation zone fluctuated between 133-172°C; after the reaction, the arsenic-removed slag and condensate were taken out. Arsenic 96.12%; take 20g of arsenic-removed slag, grind it to below 100 mesh, add 1g of sulfur powder, mix it evenly, and place it in the reactor; vacuum the reactor to 1000Pa, and heat the reactor at a heating rate of 15°C / min to 400°C, keep warm for 6h; after the reaction is over, take out the secondary arsenic removal slag after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com