Protein sequence 17E11 and use thereof
A technology for protein sequences and uses, applied in the field of gene sequences, can solve the problems of complex production process, poor specificity, and many negative effects, and achieve the effects of inhibiting tumor cell activity, overcoming poor specificity, and overcoming complex production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
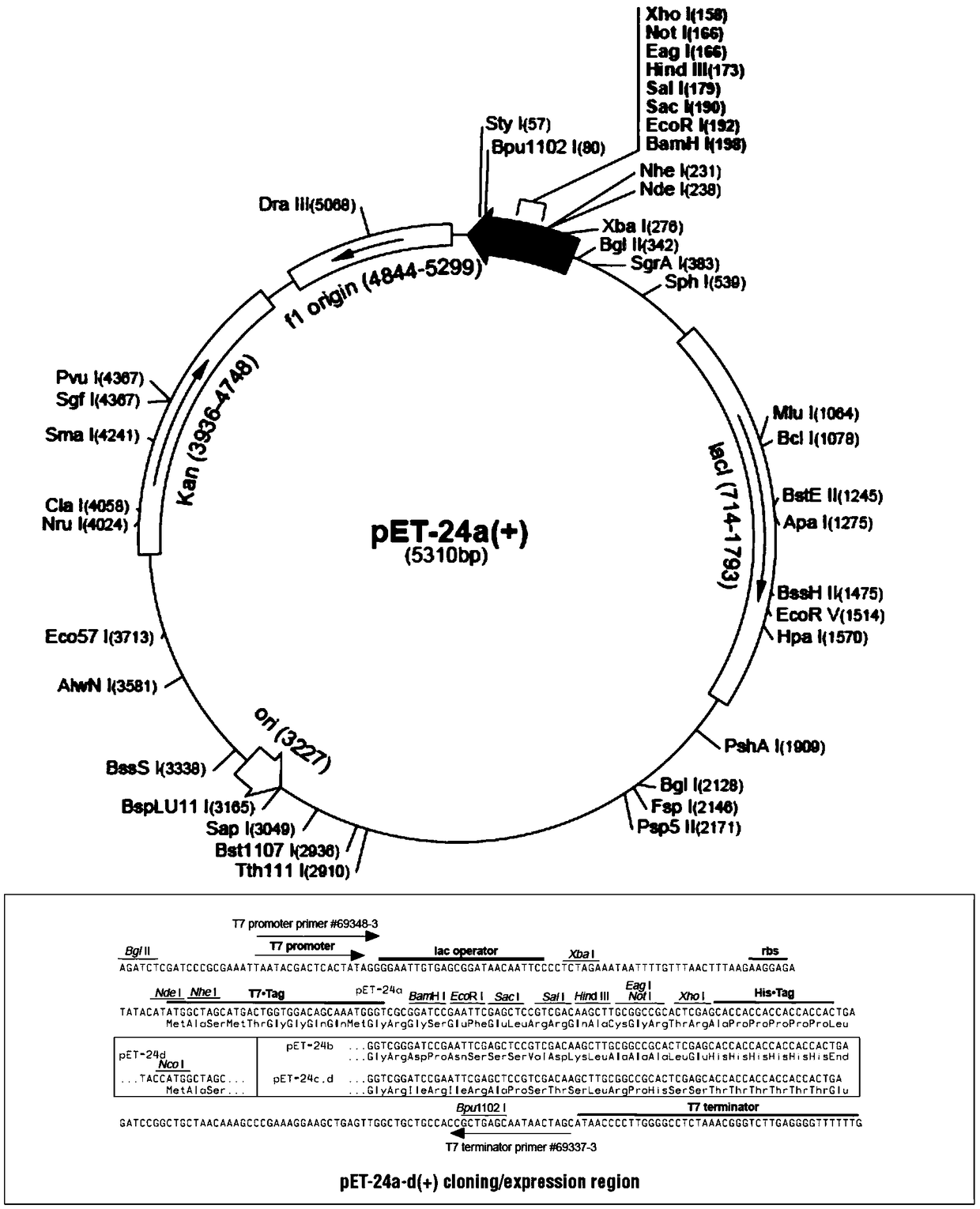
[0034] Embodiment 1: Construction of PET-24a / 17E11 expression strain
[0035] The protein sequence (SEQ ID NO:1) of 17E11 was converted into a DNA sequence and optimized (select the codons commonly used in the Ecoli system, and at the same time balance the distribution of GC bases to make them evenly distributed and reduce the probability of secondary structure formation, etc.) The following sequence (SEQ ID NO: 2), artificially synthesized SEQ ID NO: 3 after adding NdeI (CATATG) and XhoI (CTCGAG) double restriction sites at the front and rear ends of SEQ ID NO: 2 respectively (given to Suzhou Jinweizhi Biological Technology Co., Ltd.), the synthesized sequence SEQ IDNO: 3 double enzyme digestion (NdeI and XhoI) is connected to the pET-24a vector of the same double enzyme digestion, and then transferred to DH5α cells to amplify and extract the plasmid to obtain the expression vector .
[0036] Then the expression vector was transformed into BL21, and the positive strain was s...
Embodiment 2
[0037] Example 2: Expanded culture and protein purification of 17E11 expression strain
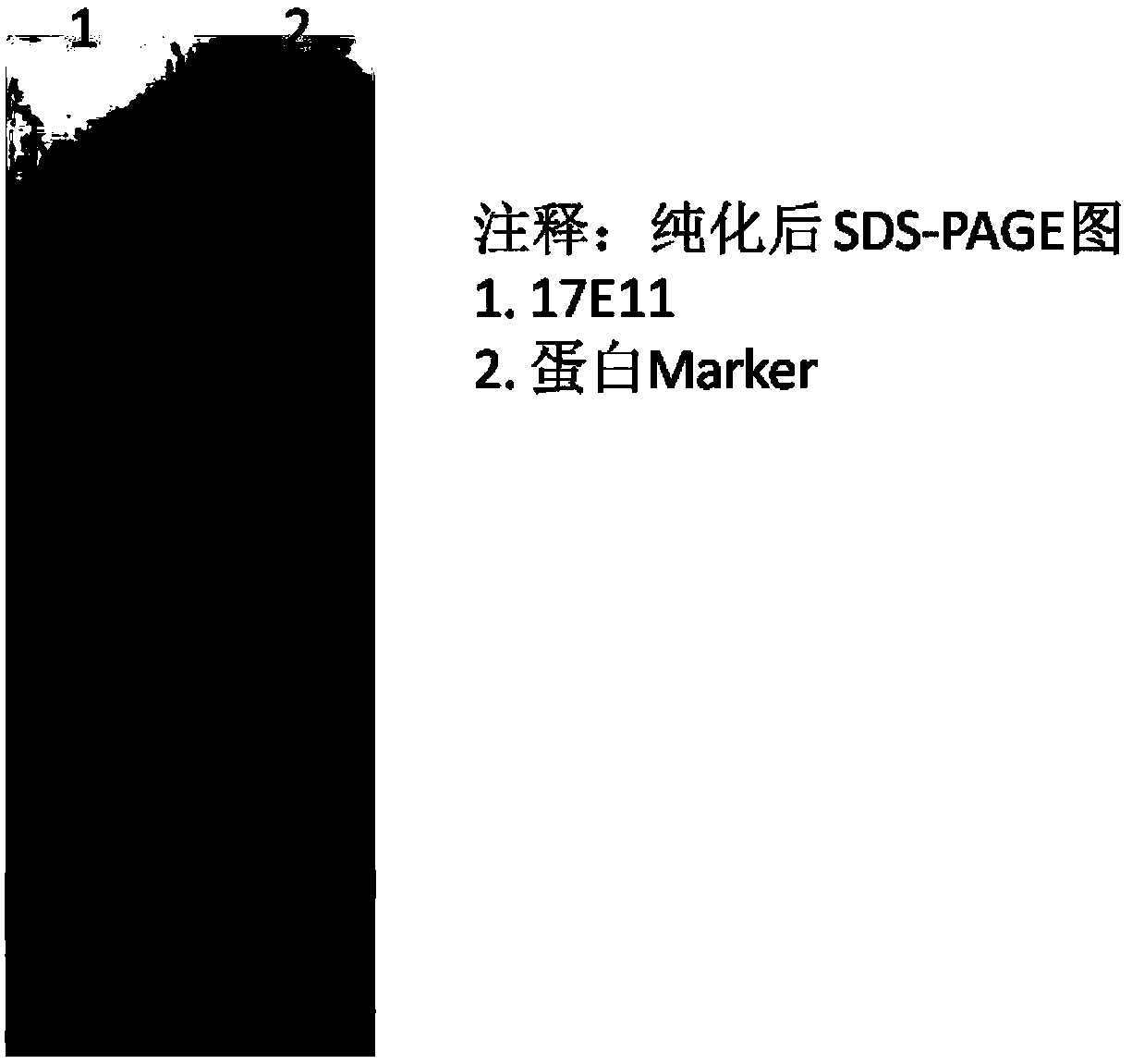
[0038] The expression strain obtained in Example 1 was recovered through the following steps, seed expansion, IPTG-induced fermentation and purification to finally obtain the target protein with a purity of >90%. The purified protein SDS-PAGE results are detailed in image 3 . Lane 1 is the purified 17E11 protein, and lane 2 is the molecular standard protein. From the SDS-PAGE electrophoresis graph, it can be seen that the purity of the target protein in the cell supernatant expressed in the 17E11 cell reached more than 90% after being purified by a nickel column.
[0039](1) Dilute the target glycerol bacterium frozen at -80°C with LB liquid medium 100,000 times, spread on the agar plate with 50 μg / ml Kan (or scrape the glycerol bacterium with a sterilized pipette tip, scratch line on the agar plate), and cultured upside down in a 37°C incubator overnight (about 16h);
[0040] (2) Pick...
Embodiment 3
[0048] Example 3: Determination of 17E11 in vitro activity
[0049] 1. FACS screening and verification of AXL high expression cell lines
[0050] Through relevant literature research, the applicant selected candidate cell lines with high expression of AXL: A549-luc, MDA-MB-231-luc, SKOV3, NCI-H1975-luc, and verified them by FACS. The specific test protocol is as follows:
[0051] 1) Cell sample preparation: collect 1×10 cells in a 2ml round bottom EP tube 6 centrifuge at 800rpm for 5min; discard the supernatant, add 1ml sterile PBS to wash the cell pellet; centrifuge at 800rpm for 5min; discard the supernatant, add 100μl sterile PBS to resuspend the cells; transfer the cell suspension to a 96-well round bottom plate.
[0052] 2) Primary antibody (Anti-AXL antibody) incubation: Anti-AXL antibody was added to the cell suspension and incubated at 4°C for 60 min.
[0053] 3) Wash Anti-AXL antibody: centrifuge the 96-well round bottom plate at 2000rpm×3min, discard the supernatan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com