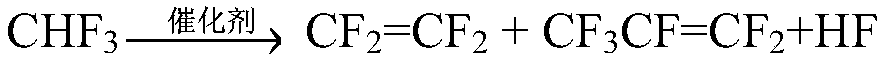Method for preparing tetrafluoroethylene and producing hexafluoropropylene by catalytic cracking of trifluoromethane
A trifluoromethane, catalytic cracking technology, applied in the field of fine chemicals, can solve the problems of further improvement of trifluoromethane conversion rate and total selectivity, increase of production cost, high reaction temperature, etc., to reduce post-processing cost and low cost , The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A method for preparing tetrafluoroethylene and co-producing hexafluoropropylene by catalytic cracking of trifluoromethane, the involved reaction equation is:
[0045]
[0046] Include steps:
[0047] (1) 5%wtCsF / Al 2 o 3 The supported catalyst was prepared as follows: 5 g of CsNO 3 Dissolved in water, prepared as 5wt% CsNO 3 Aqueous solution, ie impregnation solution; 100g of dried Al 2 o 3 Put the carrier into the impregnating solution, take it out after immersing for 20min, and dry it at 100°C for 3h to obtain the precursor; then bake it in nitrogen atmosphere at 400°C for 6h; In the reaction tube, N 2 , N 2 The flow rate is 300ml / min, and it is dried at a temperature of 300°C for 24h. Then pass trifluoromethane gas at 300°C for fluorination, with a flow rate of 6L / min, and turn off nitrogen after 2h; pass trifluoromethane alone, with a flow rate of 6L / min, and fluorinate at 350°C for 60h to finally obtain a catalyst, which is recorded as 5%wtCsF / Al 2 o...
Embodiment 2
[0055] A method for preparing tetrafluoroethylene co-production of hexafluoropropylene by catalytic cracking of trifluoromethane, different from Example 1:
[0056] The supported catalyst used was 3.6% wtLaF 3 / Al 2 o 3 , its preparation method is consistent with the preparation method of the supported catalyst in Example 1, the difference is: 3.6gLa(NO 3 ) 3 Dissolved in water, prepared as 3.6wt% La(NO 3 ) 3 Aqueous solution, i.e. dipping solution; All the other steps are identical with embodiment 1;
[0057] In step (2), the space velocity of trifluoromethane is 20h -1 ;
[0058] Other steps and conditions are consistent with Example 1.
[0059] After the cyclic reaction in this example, the conversion rate of trifluoromethane was 86%, and the total selectivity between tetrafluoroethylene and hexafluoropropylene was 97%.
Embodiment 3
[0061] A method for preparing tetrafluoroethylene co-production of hexafluoropropylene by catalytic cracking of trifluoromethane, different from Example 1:
[0062] The supported catalyst used was 1% wtPtF 4 / Al 2 o 3 , its preparation method is consistent with the preparation method of the supported catalyst in Example 1, the difference is: 1gPtCl 4 Soluble in water, prepared as 1wt% PtCl 4 Aqueous solution, i.e. dipping solution; All the other steps are identical with embodiment 1;
[0063] In step (2), the space velocity of trifluoromethane is 1000h -1 ;
[0064] Other steps and conditions are consistent with Example 1.
[0065] After the cyclic reaction in this example, the conversion rate of trifluoromethane was 87%, and the total selectivity between tetrafluoroethylene and hexafluoropropylene was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com