Acid-sensitive cyclodextrin and preparation method thereof
A cyclodextrin, acid-sensitive technology, applied in the field of medical materials, to achieve the effect of increasing water solubility, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: The acid-sensitive cyclodextrin structure of this embodiment is as follows:
[0030]
[0031] where m=6; n=0; R 1 =CH 3 , R 2 =H.
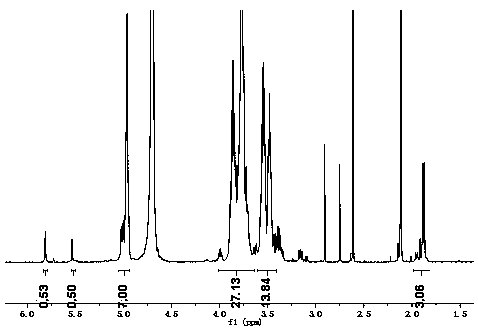
[0032] Stir and dissolve 0.4mmol of amino β-cyclodextrin with 13mL of distilled water. After mixing, add the acetonitrile solution of citraconic anhydride (3.6mmol of citraconic anhydride), stir for 10min, then add 13mmol of triethylamine solution to react, at 25℃ After the reaction was completed, adjust the pH to 6.5 with 2mol / L HCl, then slowly drop the reaction solution into methanol 10 times its volume and stir, the precipitate was precipitated, centrifugally filtered, and the filter residue was washed 3 times with methanol, at 55 Drying at ℃, the obtained white solid is the acid-sensitive amino β-cyclodextrin, and the yield is 62%. from figure 1 The proton nuclear magnetic resonance spectrum of the acid-sensitive amino β-cyclodextrin shows that the compound was successfully prepared.
[0033] Use the amine-based ...
Embodiment 2
[0034] Embodiment 2: The acid-sensitive cyclodextrin structure of this embodiment is as follows:
[0035]
[0036] where m=6; n=1; R 1 = H, R 2 =H.
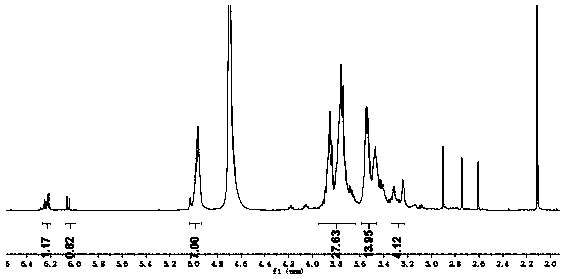
[0037] Stir and dissolve 0.4mmol ethylenediamine β-cyclodextrin with 13mL distilled water, add maleic anhydride in acetonitrile solution (maleic anhydride 4mmol) after mixing, stir for 10min, then add 15mmol triethylamine solution to react, at 40℃ Stir for 5 hours. After the reaction is completed, adjust the pH to 6 with 2mol / L HCl, then slowly drop the reaction solution into ethanol with 6 times its volume and stir, the precipitate is precipitated, centrifugally filtered, and the filter residue is washed 4 times with ethanol. Drying at ℃, the obtained white solid is acid-sensitive ethylenediamine γ-cyclodextrin, with a yield of 55%; from figure 2 The hydrogen nuclear magnetic resonance spectrum of the acid-sensitive ethylenediamine β-cyclodextrin shows that the compound was successfully prepared.
Embodiment 3
[0038] Embodiment 3: The acid-sensitive cyclodextrin structure of this embodiment is as follows:
[0039]
[0040] where m=6; n=1; R 1 = H, R 2 =CH 3 .
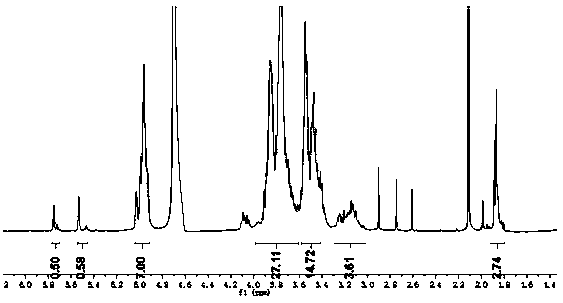
[0041]Stir and dissolve 0.4mmol ethylenediamine β-cyclodextrin with 13mL distilled water, add citraconic anhydride in acetonitrile solution (4mmol citraconic anhydride) after mixing, stir for 10min, then add 15mmol triethylamine solution to react, at 40℃ Stir for 5 hours. After the reaction is completed, adjust the pH to 6 with 2mol / L HCl, then slowly drop the reaction solution into ethanol with 6 times its volume and stir, the precipitate is precipitated, centrifugally filtered, and the filter residue is washed 4 times with ethanol. Drying at ℃, the obtained white solid is acid-sensitive ethylenediamine γ-cyclodextrin, with a yield of 50%; from image 3 The proton nuclear magnetic resonance spectrum of the acid-sensitive amino β-cyclodextrin shows that the compound was successfully prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com