Optically-controlled polymer sensitive membrane electrochemical detection method and device thereof
A detection method and a technology of polymer membranes, which are applied in the direction of measuring devices, electrochemical variables of materials, scientific instruments, etc., can solve the problems that the ions to be measured cannot be directly detected and applied, and achieve convenient operation, improved sensitivity, and strong versatility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
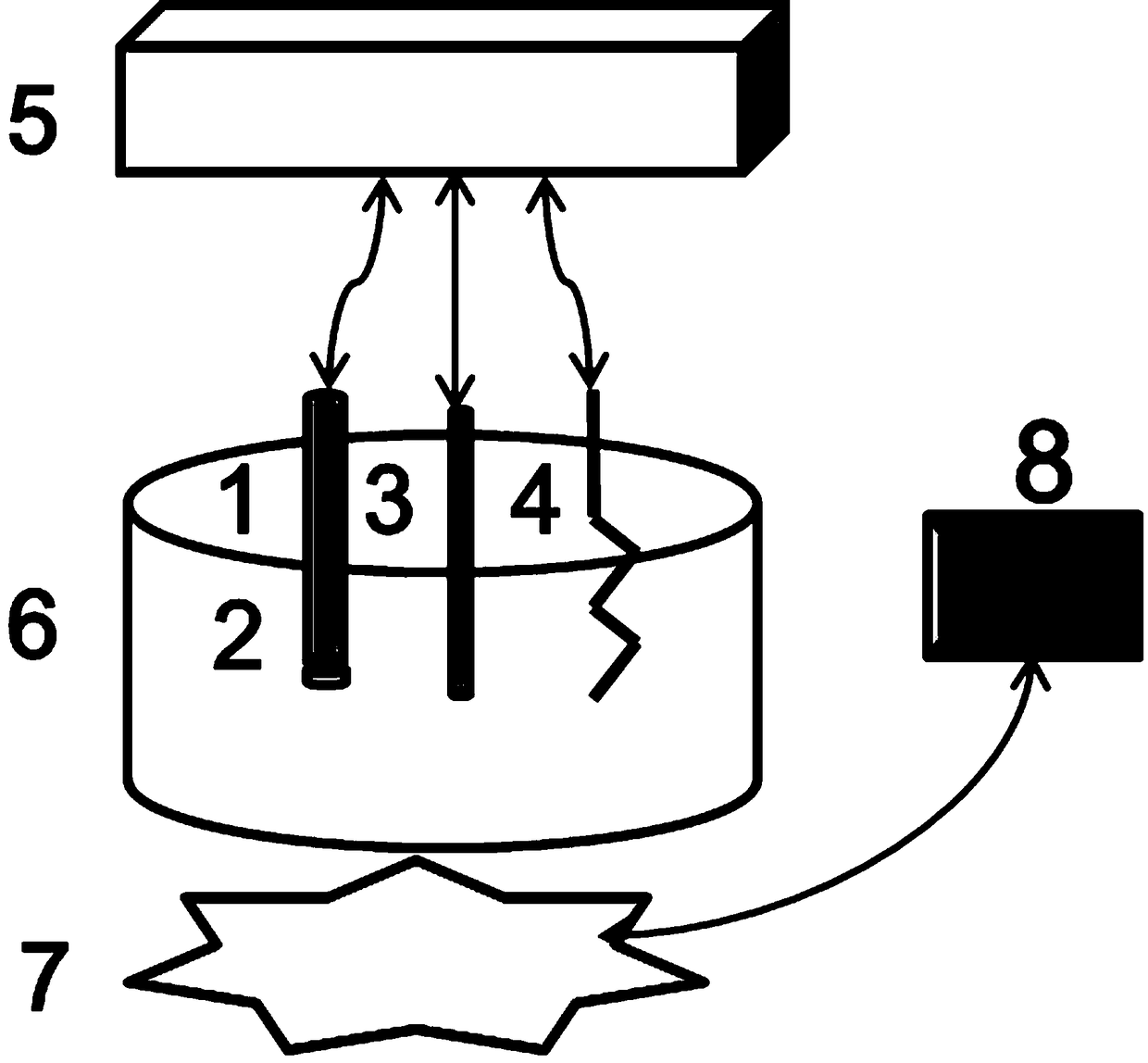
[0038] device such as figure 1 The shown includes a detection cell, an external electrochemical measuring device, and a light source; the detection cell is placed on the light source, and a working electrode, a reference electrode and an auxiliary electrode are inserted in the detection cell, and each electrode is connected to the external electrochemical measuring device through wires. connect.
[0039] The working electrode is a polymer membrane ion-selective electrode (1), with a polymer sensitive membrane (2) at the bottom; the external reference electrode (3) is silver silver chloride or saturated calomel electrode, platinum wire / piece The electrode is a counter electrode (4). The detection pool (6) holds the object to be detected; the light source is connected with an electronic relay (8) that controls its switch and illumination time through wires.
[0040] The external potential measuring device is an electrochemical workstation or an ion meter.
Embodiment 2
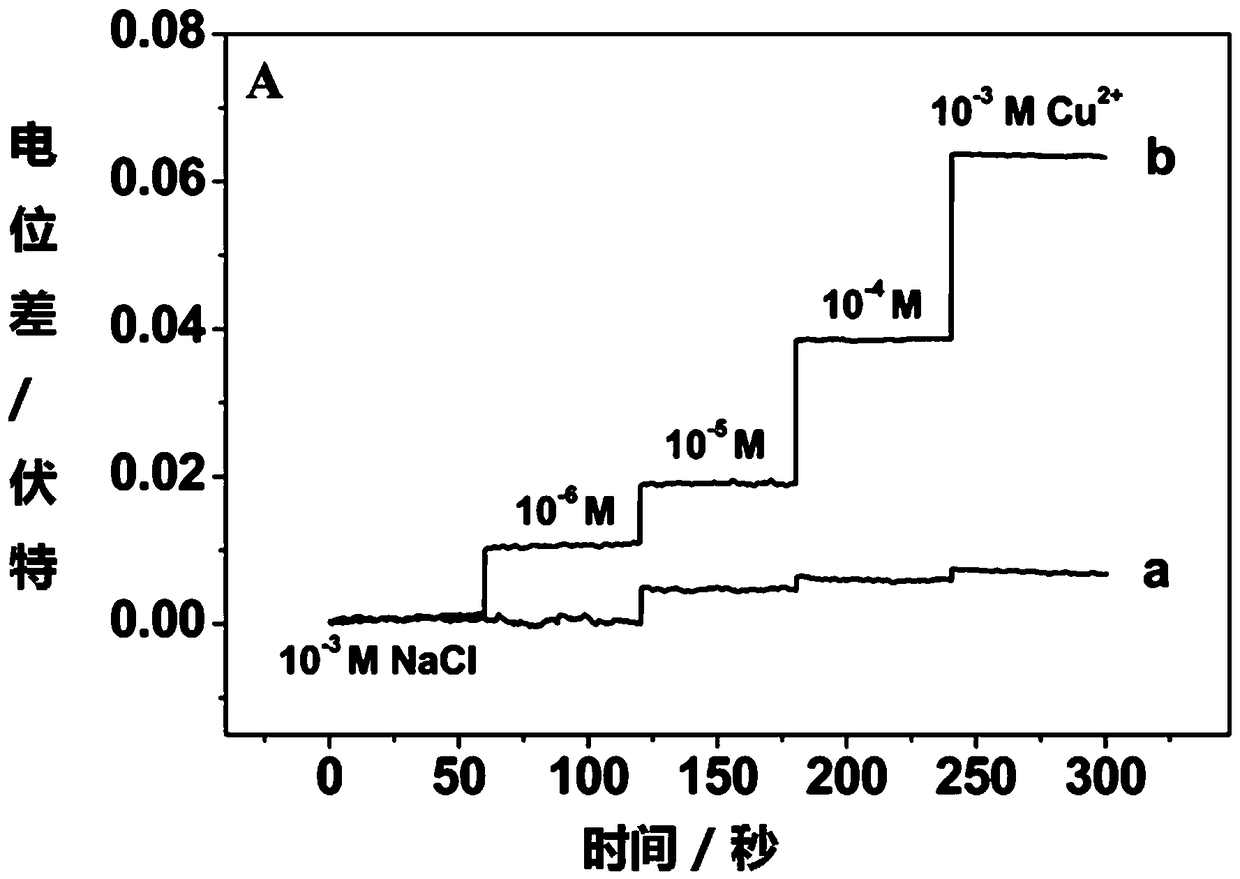
[0042] Use the above-mentioned device to detect the potential response in different concentrations of copper ions, specifically:
[0043] Preparation of polymer membrane ion selective electrode:
[0044] 1) The preparation of the electrode is
[0045] ① Obtaining the conductive layer: Electropolymerize EDOT (0.01M) and PSS (0.1M) (the molar ratio of the two substances is 8-10:1) into a PEDOT (PSS) compound under constant current conditions, and then The composite is deposited on the surface of the glassy carbon electrode to form a conductive layer. The electrodeposition method is as follows: constant current method is adopted, the current is set at 0.014mA, and the deposition time is 714 s; the thickness of the conductive layer can be controlled by adjusting the current and deposition time.
[0046] 2) Preparation of neutral electrolyte: sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate and bis(4-tert-butylphenyl)iodotrifluoromethanesulfonate in a molar ratio of 1:2 Mix...
Embodiment 3
[0053] Difference with embodiment 1 is:
[0054] A 375 nm LED (10.8 mm*10.8mm, 5.0 lm / W) array was used for light control experiments. The light time is controlled by a relay, and the light time is 30 s.
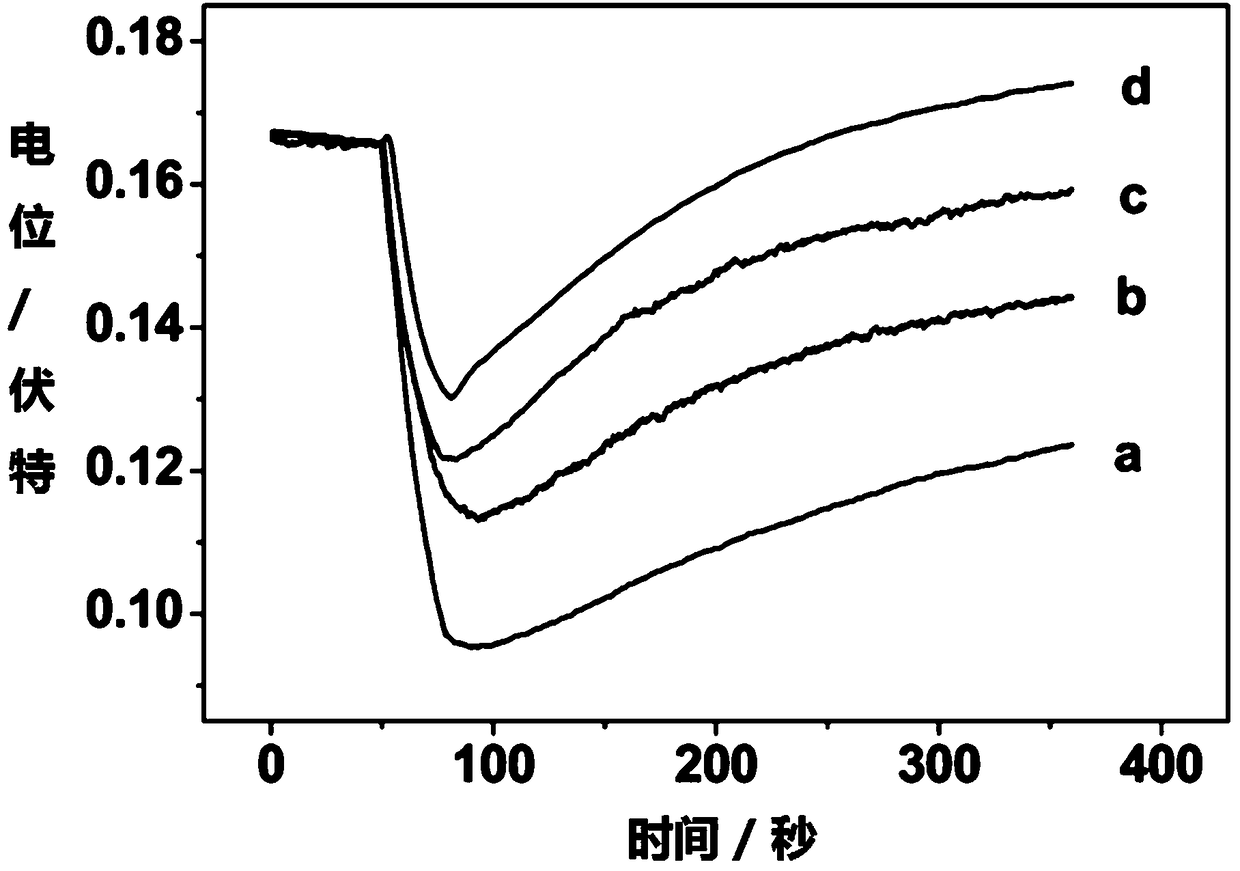
[0055] During the measurement, the open circuit potential measurement technology was used to measure the real-time potential response of the electrode when it was illuminated in different concentrations of copper ions. The results were as follows: image 3 shown. The activity of the copper ion to be measured can be determined according to the electrode potential response.
[0056] Depend on image 3 It can be seen that when light is applied, the ion exchanger in the sensitive membrane phase and the neutral electrolyte substance compounded by the photosensitive dye are photodegraded, hydrogen ions are produced, and the electrode potential decreases. When the light is over, copper ions are extracted into the sensitive membrane phase, and the electrode potential response of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com