Nanoparticle modified cross-linked polymer, polymer electrolyte, preparation method and application thereof
A technology of cross-linked polymers and nanoparticles, which is applied in the field of lithium-ion batteries, can solve the problems of low ion conductivity and complicated operation, and achieve the effects of high ion conductivity, suitable flexibility, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
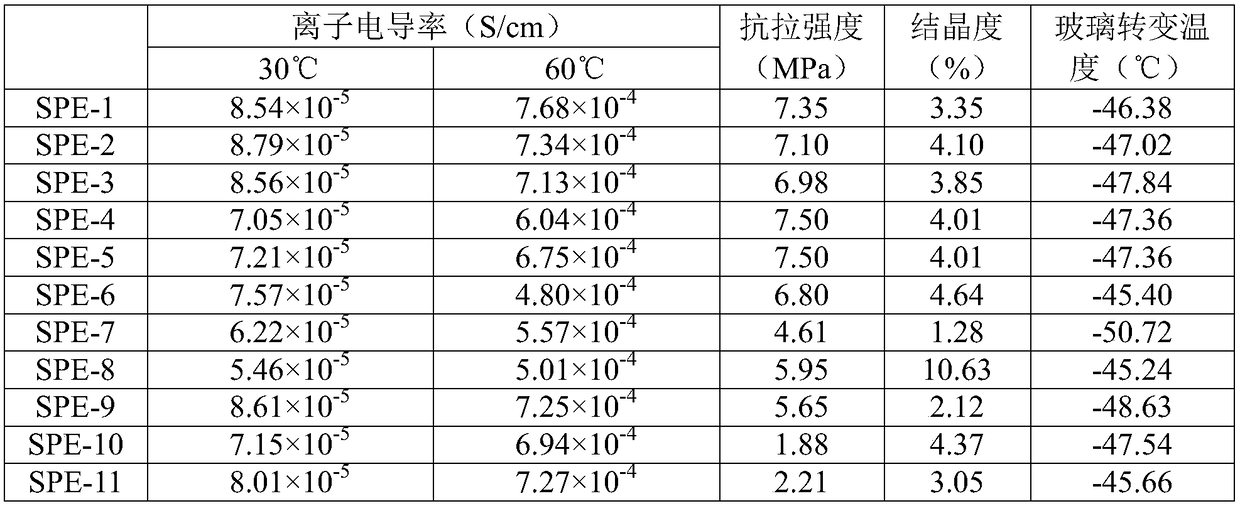
Examples
preparation example Construction
[0046] A third aspect of the present invention is a method for preparing a polymer electrolyte, the method comprising:
[0047] (1) providing a polymerization solution containing crosslinkable copolymers, lithium salts, crosslinking agents, silane coupling agent-modified inorganic nanoparticles and photoinitiators;
[0048] (2) The polymerization solution is cast and molded to obtain a semi-dry film;
[0049] (3) under ultraviolet light irradiation, the semi-dry film is cross-linked and cured;
[0050] Wherein, the crosslinkable copolymer contains the structural unit shown in formula (1), the structural unit shown in formula (2) and the structural unit shown in formula (3);
[0051] Formula 1): Formula (2): Formula (3):
[0052] Wherein, R is H or C1-C4 alkyl, L is C0-C4 alkylene or -R 1 -O-R 2 -, R 1 C0-C4 alkylene, R 2 is C0-C4 alkylene;
[0053] The crosslinking agent is one or more of the acrylate crosslinking agents containing at least two acrylate groups, an...
preparation example 1
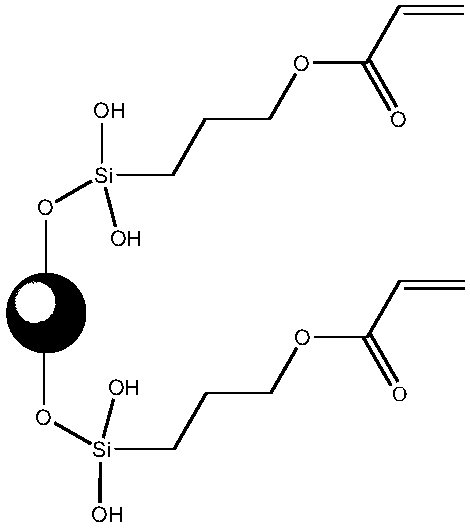
[0081] This preparation example is used to illustrate the inorganic nanoparticles modified by silane coupling agent.
[0082] Nano-SiO with a mass ratio of 1:2 2 (purchased from Beijing Deke Daojin Technology Co., Ltd., as particles with a particle size of 30nm) and 3-acryloyloxypropyltrimethoxysilane were added to ethanol (ethanol and nano-SiO 2 The mass ratio is 5:1), followed by ultrasonic dispersion for 30 minutes, and reacting at 100° C. for 12 hours to obtain inorganic nanoparticles modified by silane coupling agent C1.
preparation example 2
[0084] Nano Al 2 o 3 (purchased from Sumitomo Corporation, Japan, as particles with a particle size of 300nm) in hydrochloric acid (concentration is 0.1mol / L) to carry out surface treatment to make nano-Al 2 o 3 With hydroxyl groups on it, and then the surface-treated nano-Al according to the mass ratio of 1:3 2 o 3 with 3-methacryloxypropylethyldiethoxysilane added to isopropanol (isopropanol and nano-SiO 2 The mass ratio is 4:1), followed by ultrasonic dispersion for 35 minutes, and reacted at 110° C. for 10 hours, so as to obtain the inorganic nanoparticles C2 modified by the silane coupling agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com