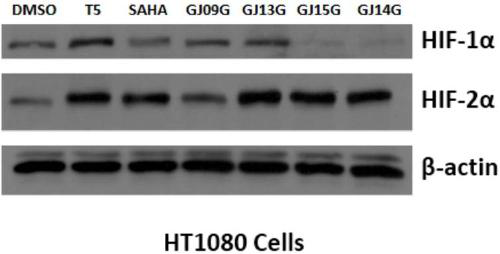
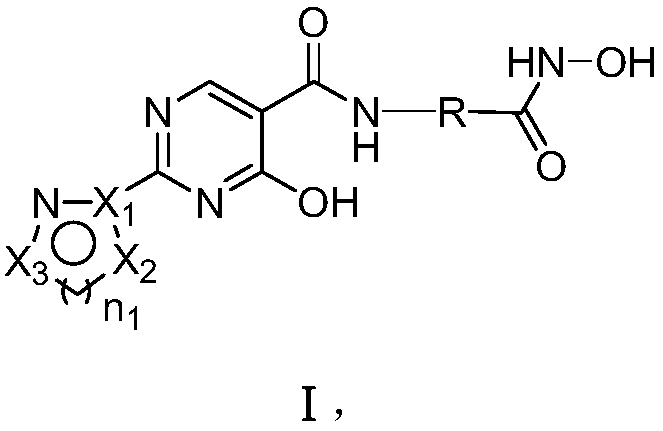
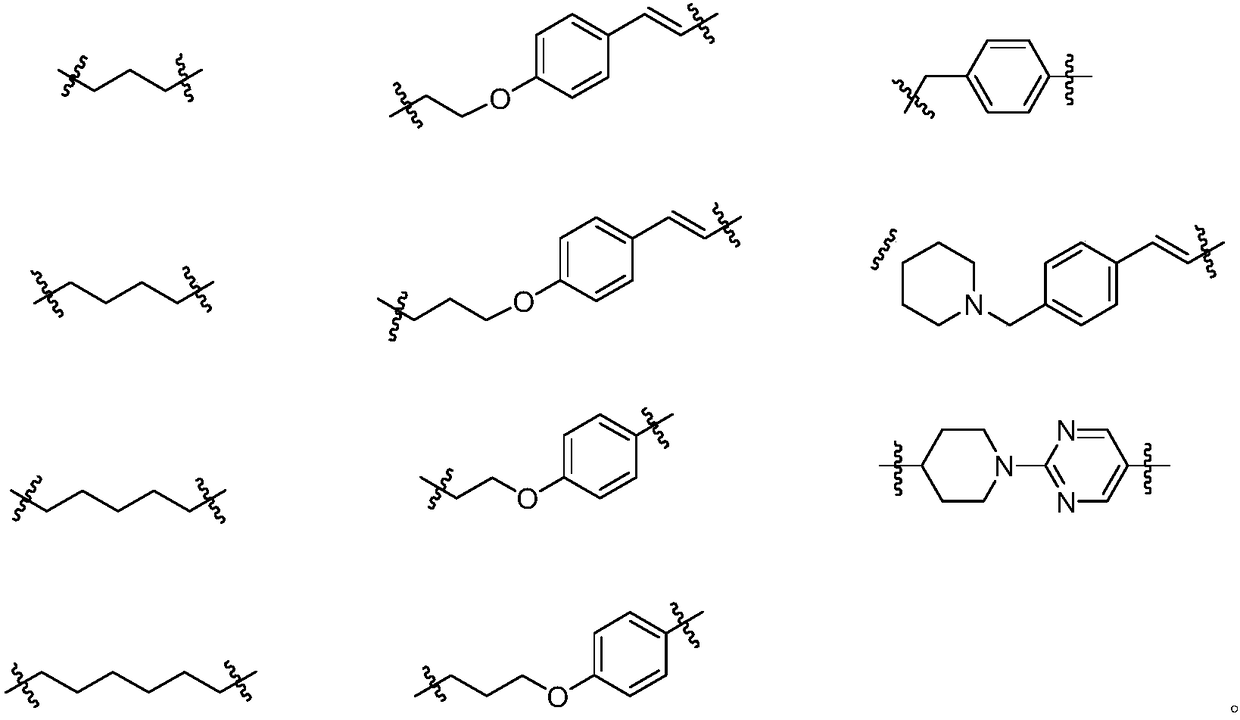
Dual inhibitor for prolyl hydroxylase and histone deacetylase, preparation method and application thereof
A technology of reaction and compound, applied in the field of application in the preparation of radiation protection drugs, can solve the problems of lack of similar compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Ethyl 4-hydroxy-2-pyrazol-1-ylpyrimidine-5-carboxylate (2)
[0061]
[0062] Add pyrazole-1-carboxamide hydrochloride (1eq) and N,N-diisopropylethylamine (2.2eq) to 5 times the volume of ethanol, stir at room temperature for 20min, add diethyl ethoxymethylidene malonate Ester (1 eq), warmed to reflux and reacted overnight. After the reaction was completed, about half the volume of ethanol was evaporated under reduced pressure, water and ethyl acetate were added for extraction twice, the organic layer was washed once with water, and the aqueous layers were combined. The pH of the aqueous layer was adjusted to 3-4, and a large amount of white insoluble matter was precipitated, which was filtered and dried to obtain a white powder of 2 with a yield of about 90.25%. m.p.162.8-163.8℃; ESI-MS(m / z): 235.0822[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ13.28(s,1H),8.63–8.56(m,2H),7.96(dd,J=1.6,0.7Hz,1H),6.67(dd,J=2.8,1.6Hz,1H),4.26(q , J=7.1Hz, 2H), 1.29(t, J=7.1Hz, ...
Embodiment 2
[0063] Example 2 Ethyl 4-(4-methoxybenzyloxy)-2-pyrazol-1-ylpyrimidine-5-carboxylate (3)
[0064]
[0065] Add 2 and anhydrous potassium carbonate (1.4eq) to 5 times the volume of N,N-dimethylformamide, stir at room temperature for 30min, add a catalytic amount of potassium iodide (0.1eq), and then p-methoxychloride Benzyl chloride was dissolved in 5 times the volume of N,N-dimethylformamide, added dropwise to the system, heated to 80°C, and reacted overnight. TLC monitoring, after the reaction is complete, add 10 times the volume of water to the system, then extract twice with ethyl acetate, combine the organic layers, and then use saturated NaHCO 3 The solution was washed twice, and the organic layer was dried over anhydrous sodium sulfate. After the solvent was completely removed under reduced pressure, the residue was slurried with methanol, and the filter cake was washed with the mother liquor. The filter cake was dried to obtain 3 as a white solid powder in a yield ...
Embodiment 3
[0066] Example 3 Ethyl 4-(4-methoxybenzyloxy)-2-pyrazol-1-ylpyrimidine-5-carboxylate (4)
[0067]
[0068]3 and sodium hydroxide (5eq) were dissolved in 10 times the volume of tetrahydrofuran / water mixed solvent (volume ratio 1:1), stirred at room temperature, and the reaction was monitored by TLC. After the reaction was completed, part of the tetrahydrofuran was evaporated under reduced pressure, and water and ethyl acetate were added for extraction once. The pH of the aqueous layer was adjusted to 3-4 to precipitate a white solid, which was filtered and dried to obtain a white powder of 4 with a yield of about 87.97%. m.p.126.3-126.5°C; ESI-MS (m / z): 327.3 [M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ13.17(s,1H),8.95(s,1H),8.74(d,J=2.7Hz,1H),7.93(d,J=1.4Hz,1H),7.50(d,J=8.6Hz, 2H), 6.93(d, J=8.6Hz, 2H), 6.64(dd, J=2.8, 1.6Hz, 1H), 5.57(s, 2H), 3.75(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com