Method for synthesizing lorlatinib
A synthesis method and technology of lorlatinib, applied in the field of synthesis of the drug lorlatinib, can solve the problems of high cost, long synthesis route, long time-consuming and the like, and achieve the effects of improved yield, simple operation and short synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
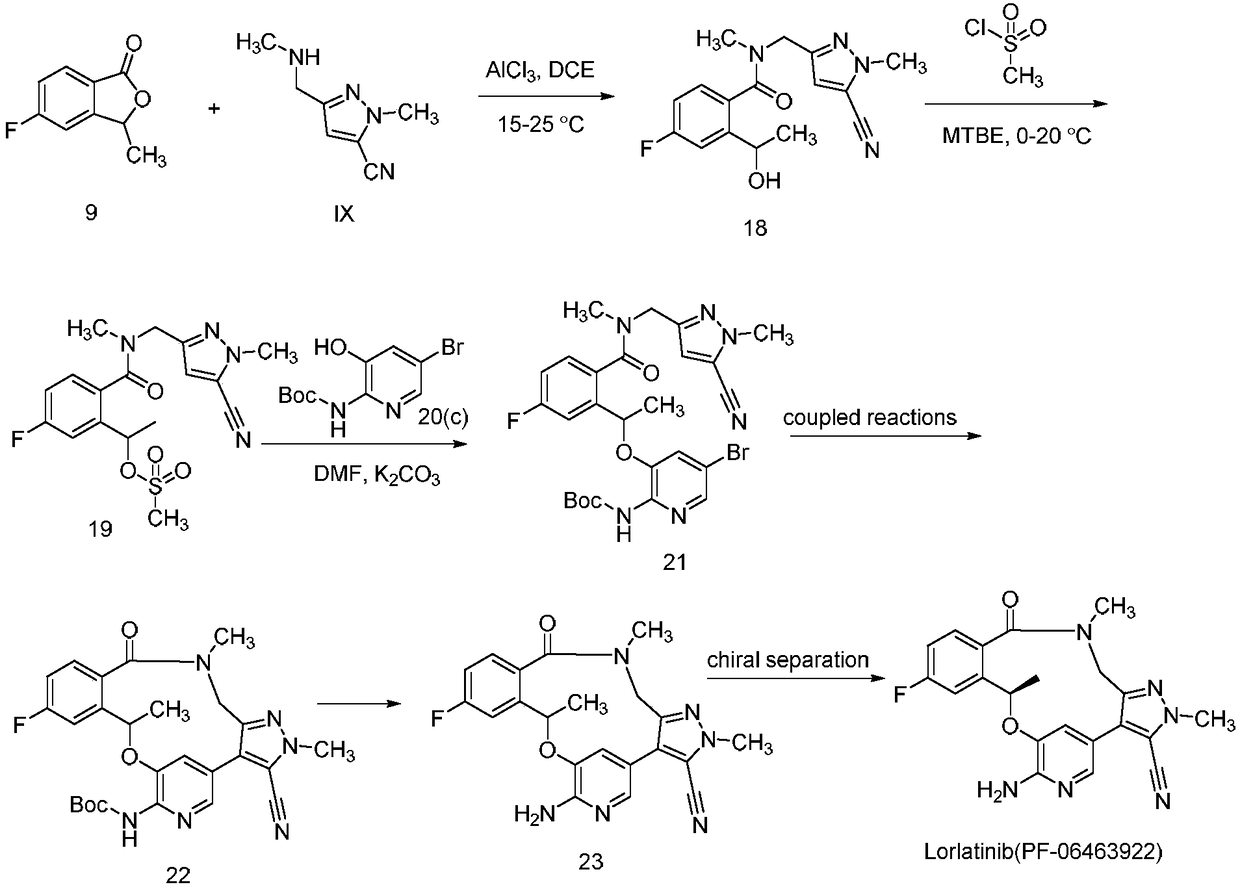
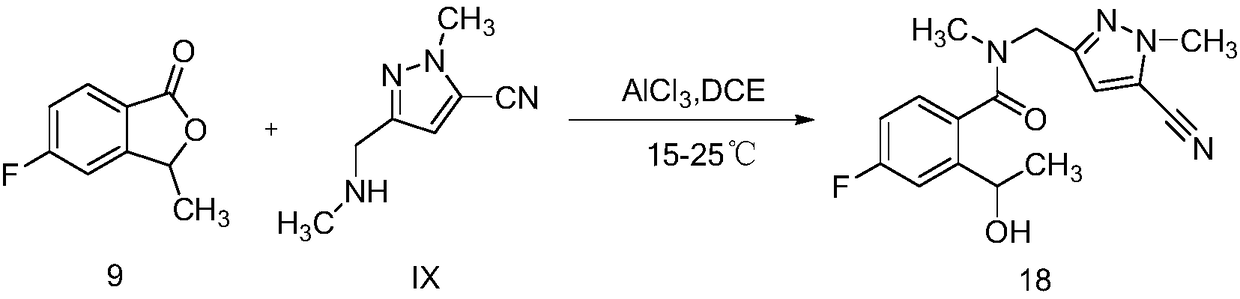
[0023] (1) N-((5-cyano-1-methyl-1H-pyrazol-3-yl)methyl)-4-fluoro-2-(1-hydroxyethyl)-N-methylbenzyl Amide synthesis
[0024]
[0025] or
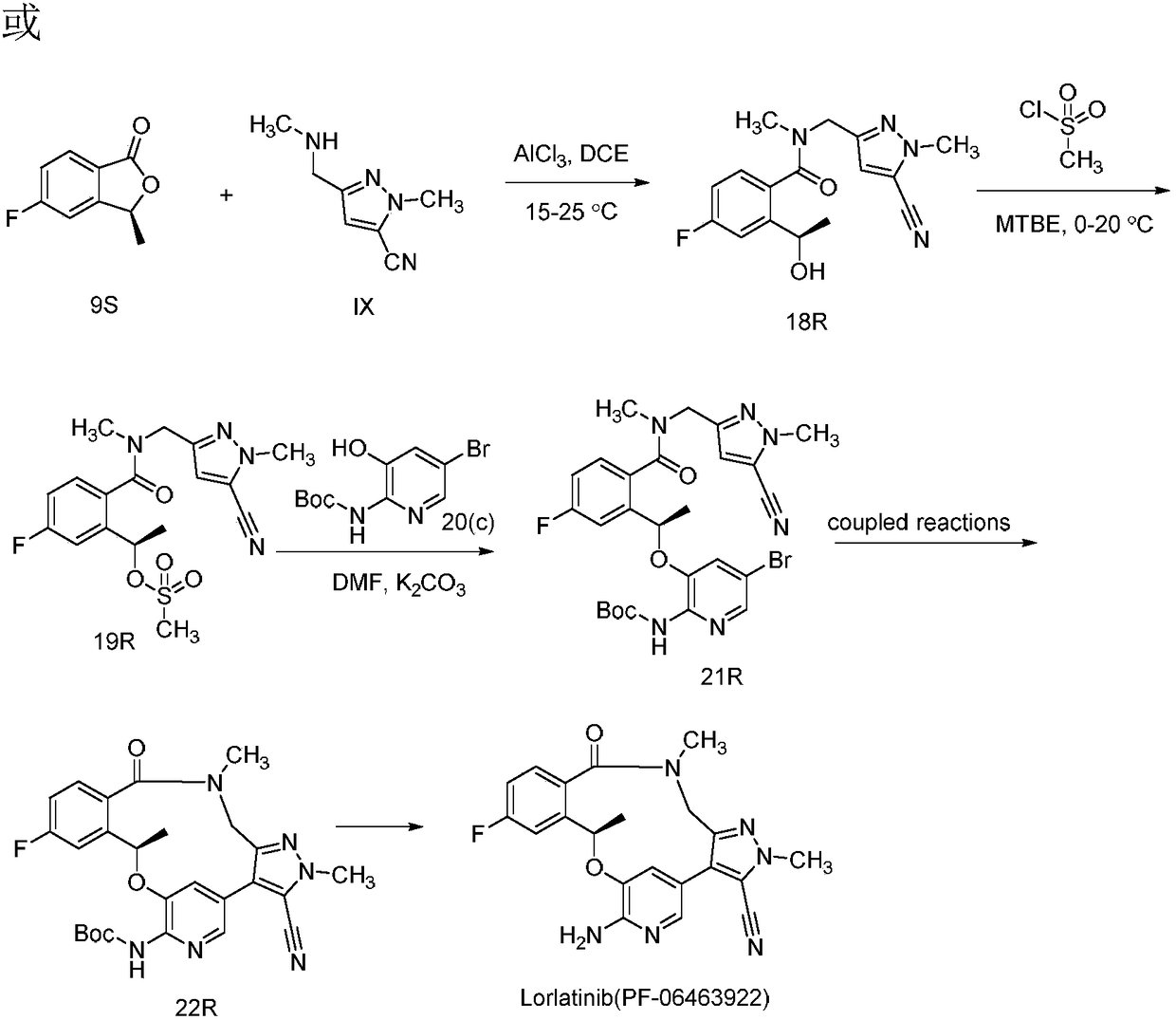
[0026] (R)-N-((5-cyano-1-methyl-1H-pyrazol-3-yl)methyl)-4-fluoro-2-(1-hydroxyethyl)-N-methylbenzene Synthesis of formamide
[0027]
[0028] 50mL three-necked bottle, equipped with a thermometer, stirring bar, and dropping funnel, dissolve 0.11g (0.79mmol) of anhydrous aluminum trichloride in 8mL DCE, and slowly add 0.23g ( 1.51mmol) 4mL of IX DCE solution, dropwise completed, reacted at 20°C for 30min, then added 0.1g (0.61mmol) intermediate (9) or (9S), reacted for 3h, quenched the reaction solution with ice water, diatoms It was filtered with soil, washed with DCM, and the filtrate was extracted with DCM, dried over anhydrous sodium sulfate, and concentrated to obtain 0.15 g, with a yield of 78.9%. Column chromatography yielded 0.12g. ESI-MS (m / z): 317.1 [M+H]+, 339.1 [M+Na]+.
[0029] (2) 1-(2-(((5-cyano-1-methyl-1H-pyrazol-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com